1 Introduction
Food is a key resource for living organisms given that energy intake regulates individual growth, survival, reproductive fitness and mortality rates [1,2]. Fish differ in their diet compositions and/or in their space/time distribution to reduce competition for resources [3]. In many cases, related species may coexist in a given natural community through several processes that make the coexistence of these potential competitors long-term sustainable [4]. Partitioning of resources involves differences in morphology both internal and external, as well as foraging behaviors [5]. Strategies used by related groups of animals to exploit resources are of great interest in ecological sciences. Many of the behavior patterns and morphological adaptations of fish species have evolved in response to the necessity of food capture, the requirements of reproduction and predator avoidance [6,7]. Most of the mechanisms that fish employ for feeding represent adaptations that enable them to take advantage of particular types of prey [8]. Research on the relationship between morphological diversity and interspecific competition for food resources is a key issue in studies involving ecomorphology and evolution [9–11]. This discipline employs a variety of morpho-biometric indexes, known as ecomorphological traits, to predict feeding patterns and habitat use of fish species [12–14].
In marine fishes, individuals tend to concentrate in estuarine and coastal areas, where they can take advantage of the high abundance of food resources [9]. Estuaries are particularly heterogeneous environments that connect freshwater and marine systems. These estuarine areas are highly productive and exhibit substantial variation in biotic (e.g., predators, competitors) and abiotic parameters (e.g., salinity, temperature) that likely influence resource availability [15]. Just as resources vary within and between estuaries, so does the feeding strategy used to acquire different prey, and this represents challenges to fishes [16]. Sciaenidae is a highly diverse family of about 283 shallow-water species, usually occurring near continental regions [17]. Several sciaenid species enter estuaries due to their high adaptability to the fluctuating physical conditions (temperature, salinity, turbidity and dissolved oxygen) in these habitats [18,19]. In Argentina, sciaenids are distributed from 34° South to 41° S and are the most abundant species in the coastal waters of Buenos Aires province [20]. Four species are commonly captured in Mar Chiquita Coastal lagoon: Cynoscion guatucupa, Pogonias cromis, Micropogonias furnieri and Menticirrhus americanus [21,22]. The later three species are commercially important resources for the coastal fisheries of Argentina, Uruguay and southern Brazil [23–26]. In Southwestern Atlantic waters, juveniles of C. guatucupa feed on mysids and penaeid shrimps, and shifts its diet to fish as it develops into adulthood [27–30]. Adults of M. furnieri based the diet mostly on decapod crustaceans and polychaetes, while juveniles feed on copepods and small polychaetes [31,32]. Trophic ecology of P. cromis has been studied in the Gulf of Mexico [33–35] and in Mar Chiquita coastal lagoon, Argentina [36], and it is composed by brachyurans and bivalve mollusks. Lastly, M. americanus has been studied in the coasts of Brazil and Mexico where it feeds on brachyurans, amphipods, polychaetes and, occasionally, mollusks [37–40], but no information on the diet of this species exists in Argentina.
Morphology is expected to influence diet of fish on a short-time scale, but on intermediate or longer time scales, diet may influence morphology through phenotypic plasticity or natural selection [13,41–45]. Giberto [46] explored the role of internal structures in the trophic relationships of the sciaenids M. furnieri, Paralonchurus brasiliensis, M. americanus, C. guatucupa, Macrodon ancylodon, and Umbrina canosai from Río de la Plata estuary. Trophic segregation between these species was explained, among other variables, by the differences in their internal morphology [46]. Although fish species present variations in their internal anatomy in relation to feeding type, these differences are small and, in general, highly invariable [18,47]. Therefore, external morphological differences can produce more meaningful results in ecomorphological studies [48]. In this context, the purpose of this study was to answer the following questions: (1) Is there any quantifiable ecomorphological variation among C. guatucupa, P. cromis, M. furnieri and M. americanus that can be related to mechanism of trophic partitioning? If so, (2) which ecomorphological attribute are correlated to differences in diet composition? Our hypothesis is that ecomorphological traits related to detection and capture of prey, vary among the four sympatric sciaenid species and that this variation can explain the differences observed in their diets.
2 Material and methods
2.1 Study area and field sampling
The study was carried out in Mar Chiquita coastal lagoon (Argentina; 37° 32′–37° 45′ S and 57° 19′–57° 26′ W) which is a 25-km-long temperate shallow estuary covering an area of 46 km2. The available literature on the fish communities of Mar Chiquita lagoon suggests that the area is likely important as nursery ground for marine and freshwater species [21,22,49]. Sampling was carried out monthly between April 2008 and June 2011, near to the mouth of the lagoon in the sea. All specimens were captured using 25 m long and 2 m wide gill-nets with 120 mm, 68 mm and 57 mm stretch mesh sizes. Due to the fishing method employed, fish were retrieved dead from the nets. Fish collection permits were given by the Ministry of Agriculture – Ministerio de Asuntos Agrarios, province of Buenos Aires (www.maa.gba.gov.ar).
The sample comprised four sciaenid species (Fig. 1). Adult specimens of sizes above length of first maturity (according to Cervigón [50], Cousseau and Perrotta [20], Macchi et al. [51] and [52]) were used for the analyses of both diet and ecomorphology, in order to avoid ontogenetic variations. A total of 289 adult specimens was measured and their stomach contents examined: 103 C. guatucupa, with a size range of 328–565 mm total length (TL) and mean ± SD of 436.3 ± 71.9 mm TL; 77 P. cromis: TL size range = 330–623 mm and TL mean ± SD = 457.9 ± 84.6 mm; 61 M. furnieri: TL size range = 332–557 mm and TL mean ± SD = 402.6 ± 58.9 mm; and 48 M. americanus: TL size range = 216–323 mm and TL mean ± SD = 279 ± 30.2 mm.

Four species of Sciaenidae captured in Mar Chiquita coastal lagoon.
2.2 Do ecomorphological traits differ between species?
Ten external morphometric traits, related to foraging activity and prey capture, were measured: standard length, body height, body width, head length, eye diameter, caudal peduncle length, caudal peduncle height, caudal peduncle width, and mouth height and width. All measurements were taken to the nearest millimeter with a digital caliper. Both inter- and intra-specific size dependent differences were eliminated following Cussac et al. [53] and Milano et al. [54]. All measurements were adjusted to mean LS using the relationship:
Based on the adjusted morphometric measurements, 8 ecomorphological attributes were calculated to each species, which were chosen according to the criteria proposed by Colborne et al. [55], Gatz [56], Gibran [4], Helfman et al. [57] and Winemiller [58]:
- • compression index (CI): body height divided by body width. Higher values indicate a laterally compressed fish and this is positively related to swimming speed;
- • relative body height (RBH): body height divided by standard length. Lower values are directly related to the capacity of making vertical turns. High RBH values suggest greater lateral maneuverability;
- • relative length of the caudal peduncle (RLP): caudal peduncle length divided by standard length. High RLP is associated with increased swimming endurance;
- • caudal peduncle compression index (CPC): caudal peduncle height divided by caudal peduncle width. Higher values of CPC are associated with a slow swimming;
- • relative head length (RHL): head length divided by standard length;
- • relative mouth height (RMH): mouth height, when fully opened, divided by standard length;
- • relative mouth width (RMW): mouth width when fully opened, divided by standard length. High values of RMH and RMW, like RHL, imply the ability to capture larger prey;
- • relative eye diameter (RED): eye diameter divided by standard length. A high RED value is directly correlated to prey detecting visual capacity.
Ecomorphological attributes were compared among species by means of Discriminant Function Analyses (DFA). This multifactorial analysis allowed the determination of which combination of variables discriminated best among species and detected which of them were the most different [59]. The assumptions of DFA were previously tested according to Zuur et al. [60].
2.3 Does diet differ between species?
The stomach contents of each specimen were removed and stored at –20 °C for subsequent analyses. Prey items were identified under a stereomicroscope to the lowest taxonomic level using reference guides [20,61]. For each individual, the dietary items were weighed (±0.01 g) and split in 7 categories for the statistical analyses: teleosts, peneids, bivalves, brachyurans, amphipods, isopods and polychaetes according to Wainwright and Richard [45].
In order to characterize the diet of the four fish species, the percentage frequency of occurrence of each prey category was calculated [62]. Discrimination among species by their diet composition was determined using a DFA [59]. To perform the DFA, the transformation log (n + 1) on the weight of each prey category found in the stomach of each specimen was used. The assumptions of DFA were previously tested according to Zuur et al. [60].
2.4 Are ecomorphological traits related to diet?
Canonical correspondence analysis (CCA) was used to assess the relationship between ecomorphological traits and diet composition [59]. This method relates two data matrices, one of ecomorphological attributes of each individuals and the other of weight of prey items from the same samples. This method also correlates the responses between them. If the correlation between the two data matrices is statistically significant, it would conclude that both are related [59].
Finally, the relation of each ecomorphological attributes and weight of each trophic item was tested with a Pearson correlation test. The Pearson correlation coefficient measures the strength of the linear relationship between two variables [60]. Correlation was considered when the slope was significantly different from 0 (P < 0.05).
3 Results
3.1 Ecomorphology traits variability
The Discriminant Analysis (DFA) among species based on the 8 ecomorphological attributes showed highly significant interspecific differentiation (Wilk's λ = 0.037; F 24, 806 = 199.07; P < 0.001) and provided three significant discriminant functions (Table 1). These three functions together correctly classified 99.31% of the 289 individuals in their respective species. The first discriminant function (DF1) contributed 88.44% of the total variation and was principally correlated with four ecomorphological attributes related with body and head shape: RMW, RMH, RHL and RED. The second discriminant function (DF2) accounted for 9.84% of the total variation. In DF2, three ecomorphological characteristics showed the greatest effect: RBH, CI and RLP. The third discriminant function (DF3) only contributed 1.72% of the total variation and showed major correlation with CPC (Table 1).
Ecomorphological attribute differences among the four sciaenids species. Standardized canonical discriminant functions 1 to 3 (DF1 to DF3), variance explained, Wilks’ Lambda, significance (P) and canonical correlation. In bold, the highest contribution of each ecomorphological attribute to the five discriminant functions.
| Ecomorphological attribute | DF1 | DF2 | DF3 |
| Relative mouth width (RMW) | –1.287 | –0.358 | –0.763 |
| Relative mouth height (RMH) | –0.452 | 0.224 | –0.542 |
| Relative head length (RHL) | 2.728 | –0.649 | 0.453 |
| Relative eye diameter (RED) | –0.154 | 0.290 | –0.512 |
| Relative body height (RBH) | 0.522 | –0.941 | –0.238 |
| Compression index (CI) | –0.677 | 0.335 | –0.002 |
| Relative length of the caudal peduncle (RPL) | 1.196 | 0.684 | –1.009 |
| Caudal peduncle compression index (CPC) | –0.068 | –0.001 | 0.555 |
| Variance explained (%) | 88.44 | 9.84 | 1.72 |
| Total variance explained (%) | 88.44 | 98.28 | 100 |
| Wilks’ lambda | 0.004 | 0.112 | 0.638 |
| P-level | < 0.001 | < 0.001 | < 0.001 |
| Canonical correlation | 0.983 | 0.909 | 0.602 |
Based on DF1 and DF2 (Fig. 2), and according to squared Mahalanobis’ distances (D2) (Table 2), four groups corresponding each to one species could be identified. Moreover, the analysis was able to distinguish the intermediate position of the M. furnieri specimens (Fig. 2).
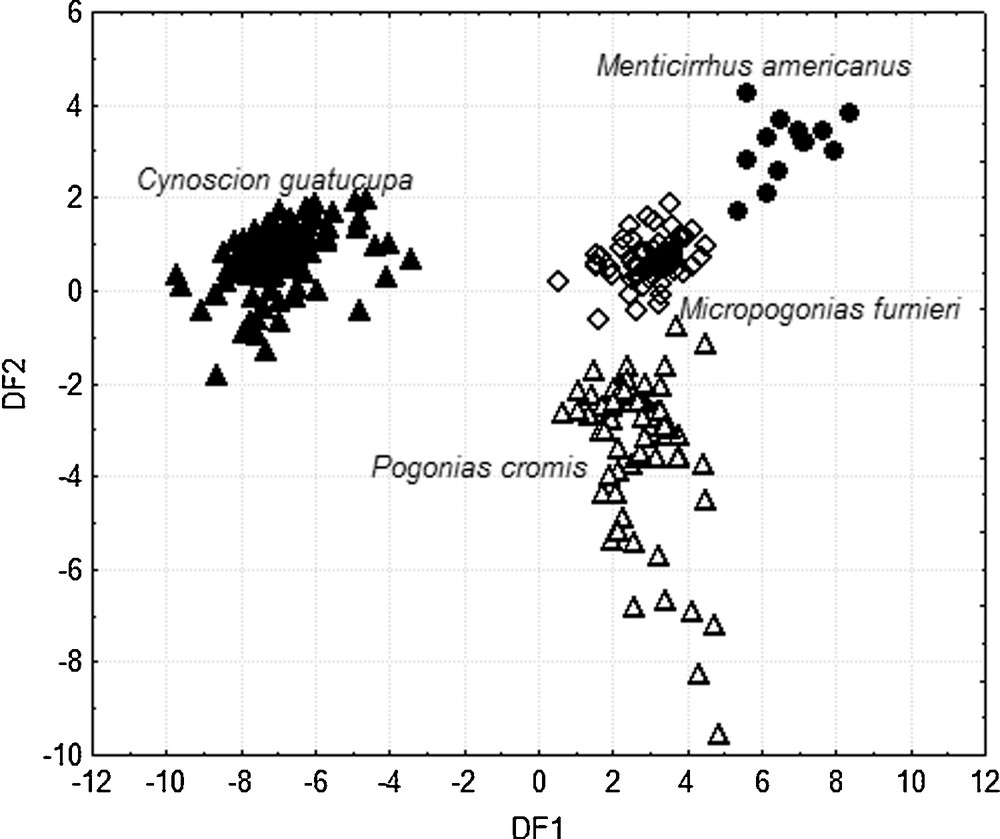
Discriminant function 1 (DF1) versus discriminant function 2 (DF2) for ecomorphological attributes of Cynoscion guatucupa (), Pogonias cromis (), Micropogonias furnieri () and Menticirrhus americanus (). Compression index (CI), relative body height (RBH), relative length of the caudal peduncle (RPL), caudal peduncle compression index (CPC), relative head length (RHL), relative mouth height (RMH), relative mouth width (RMW), relative eye diameter (RED).
Squared Mahalanobis distance (D2) among four sciaenid species, F-value to 8 and 278 d.f., and significance (P).
| Species combinations | D 2 | F-value | P-level |
| Cynoscion guatucupa × Pogonias cromis | 108.39 | 582.34 | < 0.001 |
| C. guatucupa × Micropogonias furnieri | 101.29 | 473.15 | < 0.001 |
| C. guatucupa × Menticirrhus americanus | 195.40 | 780.07 | < 0.001 |
| P. cromis × M. furnieri | 25.46 | 83.40 | 0.007 |
| P. cromis × M. americanus | 58.10 | 209.46 | 0.002 |
| M. furnieri × M. americanus | 18.51 | 76.80 | 0.006 |
The distribution of the specimens (Fig. 2) and the correlation of ecomorphological attributes (Table 1) with DF1 and DF2, allowed the identification of key ecomorphological characteristics for each species. C. guatucupa was characterized by having a laterally compressed body and head, and a relatively large eye and mouth. P. cromis was characterized by having a relatively tall body and M. americanus had a relatively long caudal peduncle. M. furnieri showed intermediate values of mouth and head size, fusiform body and intermediate values on caudal peduncle length.
3.2 Diet composition variability
In terms of occurrence, the most frequent prey in the diet of C. guatucupa were teleosts, followed by penaeids. P. cromis fed mainly on brachyurans, and less frequently on bivalves. M. furnieri presented a broader trophic spectrum, with polychaetes, brachyurans and amphipods as the most frequent prey items. Lastly, brachyurans and polychaetes were the most common items found in the gut contents of M. americanus (Table 3).
Percentage frequency of occurrence of each prey category of four sciaenid species.
| Prey items | Cynoscion guatucupa | Pogonias cromis | Micropogonias furnieri | Menticirrhus americanus |
| Teleosts | 82.91 | 11.48 | ||
| Bivalves | 46.75 | 18.03 | ||
| Brachyurans | 77.92 | 29.51 | 56.25 | |
| Peneids | 37.69 | 16.39 | ||
| Amphipods | 4.52 | 22.95 | 18.75 | |
| Isopods | 2.51 | 18.03 | 14.58 | |
| Polychaetes | 2.01 | 3.89 | 36.07 | 47.92 |
The DFA among species based on the diet composition showed a significant differentiation (Wilk's λ = 0.231; F 21, 793 = 25.11; P < 0.001) and provided two significant discriminant functions (Table 4). Predictive classification of individuals showed that 70.63% of them were correctly classified in their respective species. The DF1 accounted for 66.20% of the total variation and was associated with the consumption of teleosts, peneids and brachyurans. In turn, bivalves, polychaetes, amphipods and isopods were largely associated with DF2 that contributed 29.11% of the total variation. The DF3 only contributed 2.69% of the variation (Table 4).
Prey item differences among the four sciaenids species. Standardized canonical discriminant functions 1 to 3 (DF1 to DF3), variance explained, Wilks’ Lambda, significance (P) and canonical correlation. In bold, the highest contribution of each prey item variable to the five discriminant functions.
| Prey item | DF1 | DF2 | DF3 |
| Teleosts | 0.774 | –0.064 | 0.412 |
| Peneids | 0.494 | 0.007 | 0.315 |
| Brachyurans | –0.410 | –0.301 | 0.230 |
| Bivalves | –0.363 | –0.774 | –0.371 |
| Polychaetes | –0.144 | 0.551 | 0.118 |
| Amphipods | –0.100 | 0.541 | 0.363 |
| Isopods | –0.067 | 0.596 | 0.422 |
| Variance explained (%) | 68.20 | 29.11 | 2.69 |
| Total variance explained (%) | 68.20 | 97.31 | 100 |
| Wilks’ lambda | 0.231 | 0.359 | 0.908 |
| P-level | < 0.001 | < 0.001 | 0.063 |
| Canonical correlation | 0.866 | 0.620 | 0.302 |
Significant differences in diet composition among same species were found. C. guatucupa was separated from P. cromis and this species was clearly differentiated from M. furnieri and M. americanus. However, a separation between M. furnieri and M. americanus was not observed (Table 5). The resulting categorization of the individuals on the basis of the DF1 and DF2 showed again a remarkable separation of C. guatucupa from P. cromis and of M. furnieri and M. americanus from the other two species (Fig. 3).
Squared Mahalanobis distance (D2) among four sciaenid species, F-value to 7 and 276 d.f., and significance (P).
| Species combinations | D 2 | F-value | P-level |
| Cynoscion guatucupa × Pogonias cromis | 8.12 | 50.05 | < 0.001 |
| C. guatucupa × Micropogonias furnieri | 7.07 | 37.48 | < 0.001 |
| C. guatucupa × Menticirrhus americanus | 5.39 | 23.98 | < 0.001 |
| P. cromis × M. furnieri | 5.41 | 25.49 | 0.005 |
| P. cromis × M. americanus | 4.20 | 20.01 | 0.037 |
| M. furnieri × M. americanus | 2.32 | 8.43 | 0.071 |
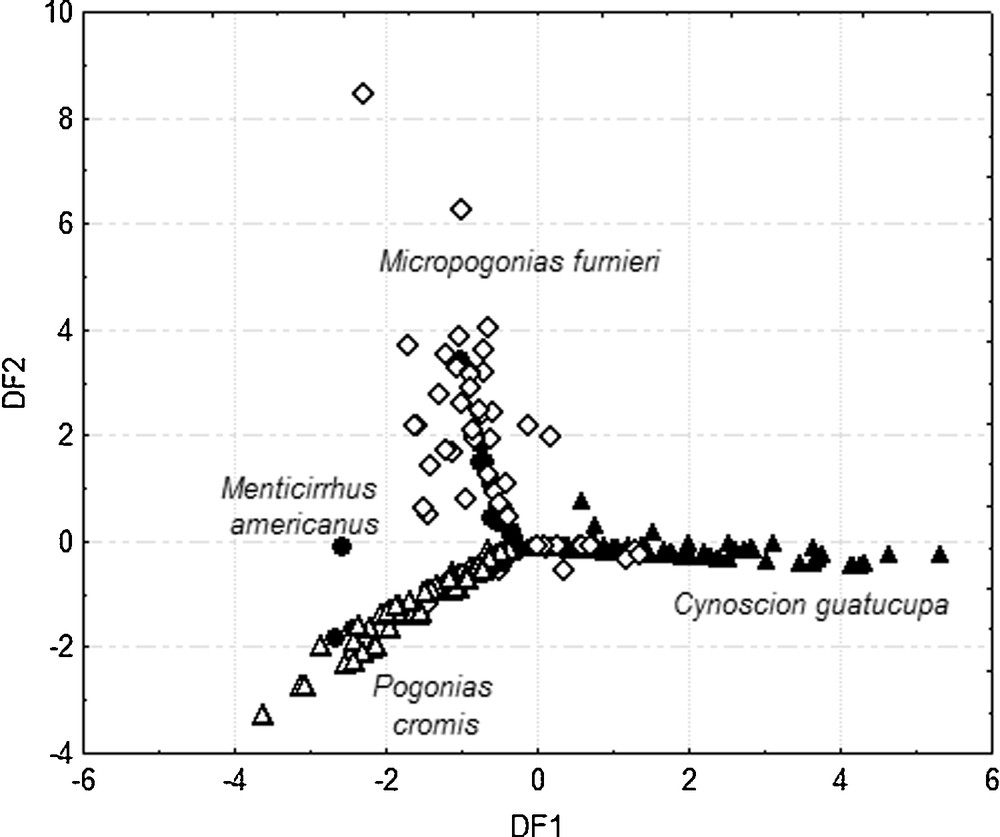
Discriminant function 1 (DF1) versus discriminant function 2 (DF2) for prey items of Cynoscion guatucupa (), Pogonias cromis (), Micropogonias furnieri () and Menticirrhus americanus ().
Overall, the DFA and D2 separated C. guatucupa, which feed mainly on teleosts and peneids, from P. cromis that forage on brachyurans and bivalves, and from both M. furnieri and M. americanus, which consume polychaetes, isopods and amphipods.
3.3 Relation between ecomorphology traits and diet
The CCA explained 95.17% of the total variability found in the relationship between ecomorphological attributes and prey items, and showed highly significant correlation between them (r = 0.843; X2 (56) = 342.79; P < 0.001).
Correlation and significance test between each ecomorphology attributes and prey items are shown in Fig. 4. The consumption of teleosts and penaeids was positively correlated with CI, RMH, RMW and RED, but negatively correlated with RBH and RLP. This indicates that species with laterally compressed body and relatively larger mouths fed on teleosts and penaeids. On the other hand, the consumption of brachyurans and bivalves were correlated negatively with RHL, RMH and RMW, and positively with RBH. These correlations indicated that fish species characterized by a tall body and a short head with a small mouth fed on brachyurans and bivalves. The consumption of amphipods, isopods and polychaetes were positively correlated with RLP and negatively correlated with CI, RMH and RMW, providing evidence that fish species with long caudal peduncle, slightly compressed body and a relatively small mouth preyed on these items (Fig. 4).

Correlation between ecomorphology attributes and prey items. P: probability of the test; CI: compression index; RBH: relative body height; RPL: relative length of the caudal peduncle; CPC: caudal peduncle compression index; RHL: relative head length; RMH: relative mouth height; RMW: relative mouth width; RED: relative eye diameter.
4 Discussion
Predation has three general components: prey search, capture and processing. Prey search may influence overall body shape to optimize mobility and energetic cost of swimming in a given habitat, while capture and processing are tightly related to head and jaw morphology [63]. In our study, C. guatucupa, P. cromis, M. furnieri and M. americanus diverged mainly in ecomorphological traits related to swimming ability, prey spotting and capture, and the size of prey that fish is able to swallow. Despite that the four species are closely related, we were able to differentiate two trophic groups. One group, characterized by demersal and pelagic feeding habits, was composed by C. guatucupa, whose diet consisted mainly of peneids and teleosts. The other group, with benthic feeding habits, was composed by M. furnieri, M. americanus and P. cromis, whose diet consisted mainly of brachyuran crabs.
Similarly with our results, Zárate-Hernández et al. [47] found that Micropogonias undulatus at the Gulf of Mexico feeds mainly on copepods and has a small mouth, whereas Cynoscion arenarius possess a much bigger mouth and feeds on larger prey, such as decapods and fish. This phenomenon is not restricted to sciaenids. The feeding habits of two sympatric pair species (Mullus barbatus–M. surmuletus and Serranus cabrilla–S. hepatus) also were related with the morphology of their feeding apparatus [7]. Hugueny and Pouilly [64] correlated the diet and morphology of 18 species of distantly related fishes from West Africa. Pouilly et al. [65] concluded that species morphology influences diet, even beyond taxonomic barriers in a study of dietary-morphological relationships of 48 species of fish in the Amazon. In summary, the published literature, coinciding with our findings, suggests that there is a significant relationship between diet and morphology leading to the conclusion that species having similar diet tend to converge for some morphological attributes, allowing for niche segregation in coexisting species.
C. guatucupa presented the greatest difference, both in ecomorphological traits and diet composition, from the other three sciaenid species. This species was characterized as a carcinophage and ichthyophage predator, with eyes and mouth bigger than the remaining species studied. Piscivorous feeding fish are hypothesized to have a slender body, which is supposed to reduce drag during fast acceleration while attacking fish prey [13]. C. guatucupa showed many of these traits, with a relatively large head and mouth, and a compressed body, indicating that it feeds on large prey, and possesses a good swimming speed [12]. Additionally to the external ecomorphological traits described in our work, C. guatucupa possess a low number of pyloric caeca, thin pharyngeal jaws with conical teeth [46]. These internal morphological traits also indicate a piscivorous feeding mode. Mouth position, in terms of whether the mouth angles up, ahead, or down, also correlates with trophic ecology in many fishes [57].
As other congeners (e.g., C. regalis and C. nannus), C. guatucupa has a terminal mouth (which means that the body ends in a mouth that opens forward), with a prominent lower jaw, canine-like teeth and a pair of big fang-like canines on the upper jaw's frontal region, that allows for an adequate prey capture [50]. Predatory fish are faced with a tradeoff between mouth size, and swimming speed and agility, which results in morphological and dietary differences [4]. Small fishes with fairly hydrodynamic bodies, forked tails, limited dentition and protractile mouths, that form a circle when open, are in all likelihood zooplanktivores. Conversely, large robust deep-bodied fishes with long jaws studded with sharp teeth, for holding prey, and with broad tails are piscivores [57]. We have observed such phenomenon in C. guatucupa: adult individuals had a more compressed body, allowing a faster and more agile swimming, therefore enabling them to capture fish, a prey that was not eaten by juvenile individuals [27].
P. cromis is found on the other side of the spectrum, exhibiting all the typical morphological characteristics of a benthic feeder, i.e. small mouth, short head, and a relatively deeper body that allow these fish to better survey the seabed [4]. Fishes that feed on hard-bodied prey, such as mollusks and crabs, often have teeth and jaw characteristics that enable the activities of capturing and processing prey, respectively [57]. P. cromis presents numerous small villiform teeth [66], subterminal mouth [20] and sensitive barbs [67]. It also has a tall head to accommodate the pharyngeal muscles. In addition, the presence of mollariform teeth that are located posteriorly in pharyngeal jaws, are used to crush and grind hard-shelled prey [68]. Accordingly, we found that P. cromis feeds mainly on mollusks and crabs, as its morphological characteristics would predict.
M. furnieri and M. americanus preyed on a variety of pelagic, demersal and benthic organisms. Both presented a set of morphological characteristics that are intermediate between C. guatucupa and P. cromis (i.e. smaller head and mouth than C. guatucupa and shorter body than P. cromis). The morphology for M. furnieri and M. americanus can be explained on the basis of their diets, which vary among prey with different habitats, as was observed in other studies [31,32,37,39,69] and also supported by their similar internal morphology (i.e. number of pyloric caeca and strength pharyngeal jaws) [46]. According to Horn [70], generalist species have broad morphological variations, probably related to the lack of specialization that characterizes them. Along its ontogenetic development, these species must feed on planktonic organisms and then shift their spectrum to demersal and benthic prey, with a versatile morphology in order to capture both infaunal and mobile prey [46]. Other sciaenid species also switch from different type of resources, presenting several strategies and shapes along their ontogenetic development, from juveniles to adults [18]. The relatively long caudal peduncle of M. americanus is an unexpected ecomorphologic trait because it is neither an active swimming predator nor it feeds on large prey [37]. Consequently, this morphological trait did not present any correlation with the diet of this species.
In conclusion, this study has shown that the four sciaenid species studied can significantly partition the food resources, even though they completely overlap spatially. These sciaenids presented divergent ecomorphological traits, which could minimize competition with each other, representing an evolutionary advantage [71]. Evolutionary pressure operates on the morphology, generating adaptations that make resource partitioning possible, thus reducing competition [12]. Differences in their ecomorphological traits appear to correlate closely with diet and consequently could explain this trophic segregation. This seemingly contribute to the coexistence of the adults of these sympatric fish species in Mar Chiquita coastal lagoon, as well as in other regions.
Acknowledgments
The authors would like to thank the Laboratorio de Biotaxonomía Morfológica y Molecular de Peces, Universidad Nacional de Mar del Plata team for their help in obtaining samples analyzed in this study. We are also grateful to Dr. Philippe Béarez, Dr. Thomas Munroe and the anonymous referees for their helpful comments that greatly improved early drafts of the manuscript. This work was funded by grants from Universidad Nacional de Mar del Plata (grant number EXA 490/10) and CONICET (grant number PIP 0942). Gabriela E. Blasina and Juan M. Molina were supported by CONICET fellowships.


