1 Introduction
Metallothioneins (MTs) are small, intracellular heavy-metal-binding proteins involved in metal detoxification and protection against oxidative stress [1,2]. Ubiquitously present in living organisms [3,4], the mammalian MT family falls into four subgroups: MT1, MT2, MT3, and MT4 [5]. MT1 and MT2 encode through a series of gene duplication events for multiple isoforms present in almost all tissues, while MT3 and MT4 evolved in a single form to accomplish specific roles in brain and epithelium, respectively [6–8]. In the mammalian brain, MT1 and MT2 are preferentially expressed in astrocytes and activated microglia [9]; MT3 is expressed predominantly in neurons and, to a lesser extent, in astrocytes [10].
It has been demonstrated that MT1 and MT2 protect the central nervous system (CNS) in response to experimentally induced injury [11] and following viral infections [12], whereas MT3 neither affects inflammatory responses nor plays an important antioxidant role [11]. Indeed, the MT3 functional role and regulation in CNS is still a matter of debate [10,13]. MT3 is known as a growth inhibitory factor able to inhibit the survival and neurite formation in cultured neurons in vitro [14]. MT3 mRNA and protein are upregulated following brain injury [15] and downregulated in Alzheimer's disease (AD) [14,16].
The known functions of MTs include metalloregulatory roles in cell growth and differentiation [17], so it is not surprising that most mammalian tissues contain age-related basal levels of MTs, with the major amount found in developing cells [18]. Enhanced synthesis of MTs is also observed in rapidly proliferating tissues, stressing their crucial role in normal and neoplastic cell growth [18–20]. However, the MTs’ role in aging is controversial: in mammals, MT expression has been found to increase in aged kidney [21], whereas decreased significantly with aging in the skin [22].
Aging is a major risk factor for brain neurodegenerative disorders, including cerebrovascular disease, AD, Parkinson's disease (PD) and cancer [23–26]. Brain aging is accompanied with molecular, functional and genetic changes, leading to increased susceptibility to diseases and cognitive impairments. As it has been proven in the mouse, MTs downregulation results in a progressive neurodegeneration, leading to early aging, morbidity, and mortality [27]; the neurodegenerative alterations are attenuated by MTs overexpression, suggesting the neuroprotective role of MTs in aging [27]. These studies, however, did not include the identification of the MT isoforms functionally involved.
In this study, by using real-time PCR analysis, we examined and compared the expression of MT1/2 and MT3 genes during aging in the rat brain. In particular, we evaluated the MT1/2 and MT3 expression profiles in the cerebral cortex and hippocampus of pubertal (2 months), adult (4 and 8 months), and middle-aged (16 months) rats.
Our results demonstrate an age-related expression of the MT3 gene, thus, suggesting its implication in physiological changes associated with aging.
2 Materials and methods
2.1 Animal and experimental design
Male Wistar rats (Charles River, Calco, Como, Italy) of 2 months of age (adolescent), with the same starting body weight (160 ± 10 g), were individually caged in a temperature-controlled room (23 ± 1 °C) submitted to a 12-h light/12-h dark cycle. Animals were housed in the Animal Care Facility at the Department of Biology, with ad libitum access to water and to a standard diet (Mucedola 4RF21; Settimo Milanese, Milan, Italy) up to 4 (social maturity, group 1), 8 (adulthood, group 2), or 16 (aged, group 3) months. Eight animals of each group were anesthetized with chloral hydrate (40 mg/100 g body wt) and killed by decapitation. Group 4 was comprised 8 adolescent rats sacrificed four days after their arrival in the animal facility. The brains were quickly removed and the cerebral cortex and hippocampus were dissected on ice. Samples of each brain region were snap frozen in liquid nitrogen immediately and stored at −80 °C for subsequent RNA isolation. The livers of the rats belonging to group 4 were also collected, frozen and stored at –80 °C for RNA isolation. The protocols for animal care and use were approved by the Scientific Ethics Committee on Animal Experimentation of the University Federico II of Naples. All experimental animal procedures were carried out in compliance with the national guidelines for the care and use of research animals (D.L.116/92, implementation of EEC directive 609/86). All efforts were made to minimize animal suffering and to reduce the number of animals used.
2.2 RNA purification and cDNA synthesis
Total RNA was extracted according to the TRI-Reagent (Sigma–Aldrich) protocol. The quality of each total RNA was checked by electrophoresis on 2% agarose gel stained with ethidium bromide and by measuring the optical density at 260/280 nm. A ratio of 1.8–2.0 was accepted for further reverse transcription. The QuantiTect Reverse Transcription Kit (Qiagen) was used for the removal of genomic DNA contamination and for the subsequent cDNA synthesis. Approximately 1 μg of total RNA was used, according to the kit's protocol.
2.3 Quantitative real-time PCR analysis
The real-time PCR reactions were carried out in quadruplicate in an Applied Biosystems 7500 Real-Time System by using the Power SYBR Green Master Mix PCR (Applied Biosystems) following procedures recommended by the manufacturer. Each SYBR Green reaction (total volume: 20 μL) contained 12 μL of real-time PCR Master Mix, 1 μL of each of the forward and reverse primers (10 μM), 2 μL of 1:1 diluted cDNA, and 4 μL of nuclease-free water. Nuclease-free water for the replacement of the cDNA template was used as a negative control. For the internal standard control, the expression of β-actin gene was quantified. Primer sequences were designed using Primer Express software (Applied Biosystems). A single pair of specific primers for both MT1 and MT2 isoforms was designed on the nucleotide sequences of Rattus norvegicus MT1 (NM 138826.4) and MT2 (NM 001137564.1). Specific primers for MT3 were designed on the R. norvegicus template NM 053968.3, choosing the most divergent sequence tracts rather than MT1/2 isoforms. β-actin primers were designed on the exon junction 75/76 (forward primer) on R. norvegicus template NM031144.2. All the primers used in real-time PCR analysis are listed in Table 1. PCR was performed under the following conditions: holding stage of 95 °C per 10 min; cycling stage (45 cycles): 95 °C × 10 s – 60 °C × 10 s – 72 °C × 10 s; melting stage: 95 °C × 5 s – 65 °C × 1 min – 95 °C × 30 s – 40 °C × 30 s. The melting curve analysis of PCR products was performed in order to ensure gene-specific amplification. Changes in the gene expression relative to the different samples were calculated according to the standard 2−ΔΔCt method described by Livak and Schmittgen [28].
Specific primers used for semiquantitative real-time PCR analyses.
| Name | Nucleotide sequence (5′–3′) | Length | Direction |
| MT1/2_F | ATGGACCCCAACTGCTCCTG | 20-mer | Sense |
| MT1/2_R | CTTTGCAGACACAGCCCTGGG | 21-mer | Antisense |
| MT3_F | CCCCTGTCCTACTGGTGGT | 19-mer | Sense |
| MT3_R | CTGCATTTCTCGGCCTTG | 18-mer | Antisense |
| β-act_F | ACCCGCCACCAGTTCGCCAT | 20-mer | Sense |
| β-act_R | CGGCCCACGATGGAGGGGAA | 20-mer | Antisense |
2.4 Statistical analysis
Data are presented as mean ± standard error of the mean (SEM) from four separate experiments in each sample. Statistical analyses were carried out by StatView software (Altura Software, Inc.). The differences between the mean values were analysed by one-way analysis of variance (ANOVA) followed by Fisher's LSD test. The differences were considered significant when P < 0.05.
3 Results
3.1 Expression of MT isoforms in liver and brains of adolescent rats
As a preliminary step, we firstly decided to compare the relative abundance of MT1/2 and MT3 transcripts in the liver, the cerebral cortex, and the hippocampus of adolescent rats. As expected, MT1 and MT2 are the major forms in the liver, whereas MT3 is the most represented isoform (Fig. 1) in the two areas of the brain, with the level of the cortex higher than that of the hippocampus. However, a significant amount of MT3 transcripts was also detectable in the liver, as well as lower levels of MT1/MT2 isoforms were found both in the cortical and hippocampal areas (Fig. 1).
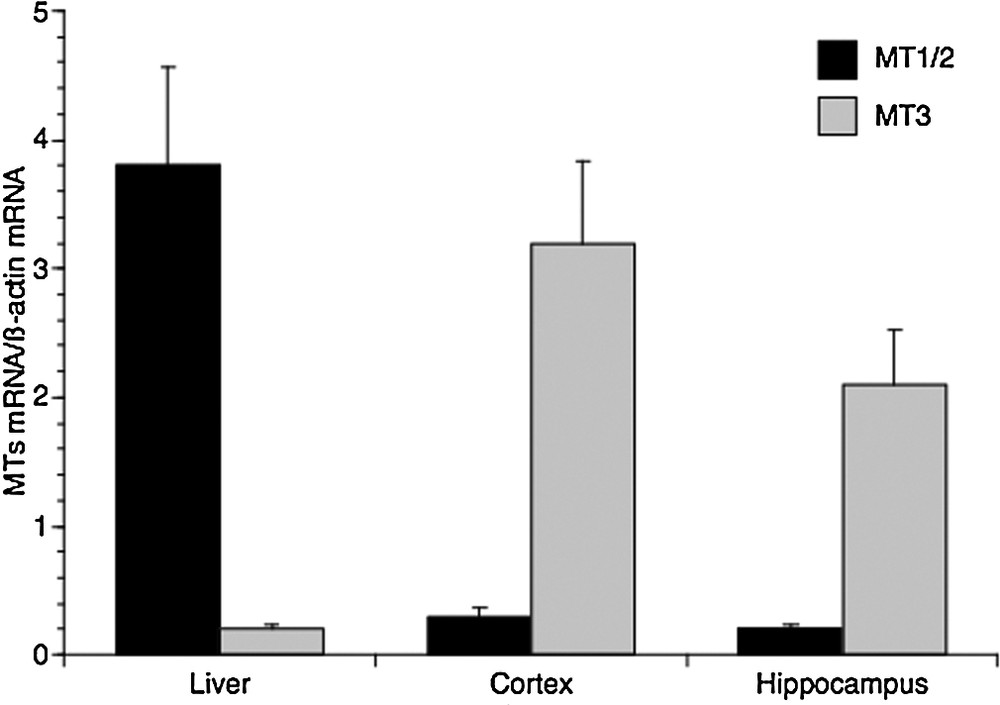
Real-time PCR analysis of metallothionein isoforms expression in liver, cerebral cortex and hippocampus of 2-month-old rats. MT1/2 and MT3 mRNA levels are shown relative to β-actin mRNA. The data represent the mean ± SEM (eight rats per group).
3.2 Expression of MT isoforms in the aging brain
Once the MTs gene expression profile in adolescent (2-month-old) rats established, we carried out the study evaluating MT1/2 and MT3 mRNAs levels in the hippocampus and the cortex of adult (4- and 8-month-old), and middle-aged (16-month-old) rats. Real-time PCR revealed a huge increase in MT3 expression in both regions of the brain, in aged rats. In particular, the level of this transcript increased by 16 times compared to the 2-month-old rats in the cerebral cortex (Fig. 2a) and by about 14 times in the hippocampus (Fig. 2b). Interestingly, we did not observe a linear increase in MT3 transcripts with the age from 2 to 8 months in both the hippocampus and the cortex; on the contrary, a slight but significant decrease in MT3 transcripts was observed in adult rats when compared with young rats (Fig. 2).
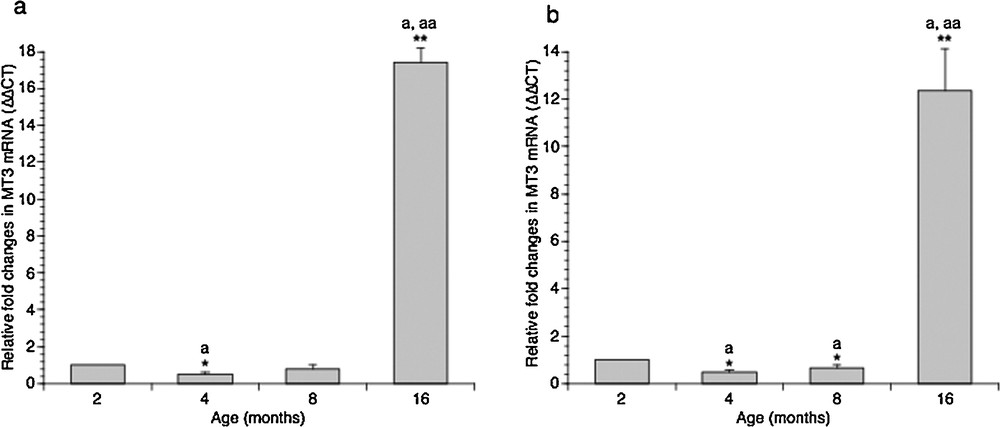
Effect of aging on MT3 gene expression in rat cortical (a) and hippocampal (b) areas. The MT3 mRNA expression was normalized to that of β-actin mRNA and converted in fold change, compared with 2-month-old rats. The data represent the mean ± SEM (eight rats per group). Significance of differences is shown. *P < 0.05; **P < 0.001. aSignificance vs. 2 months; aaSignificance vs. 4 and 8 months.
As regarding MT1/2 expression in brain areas, real-time PCR analysis demonstrated that the amount of these transcripts decreased in adults (4- and 8-month-old rats), and increased slightly only in aged rats (16 months), reaching more or less the level measured in 2-months rats, both in cortical (Fig. 3a) and hippocampal (Fig. 3b) areas.
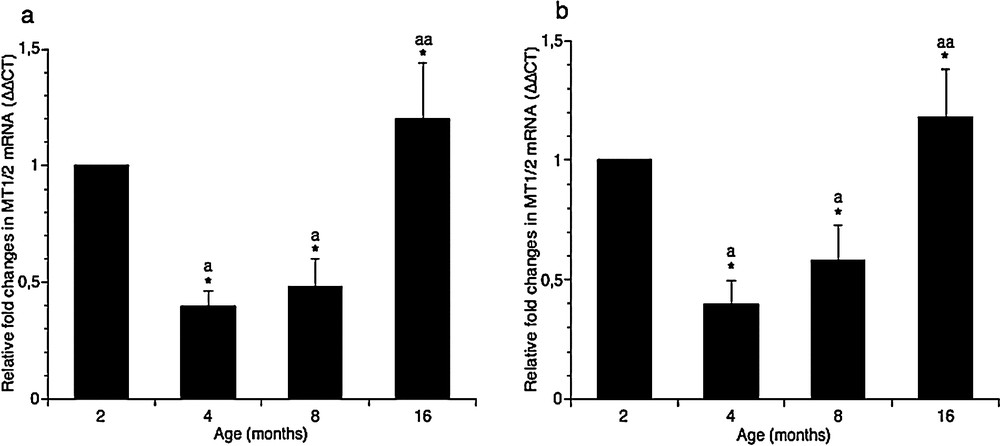
Effect of aging on MT1 and MT2 genes expression in rat cortical (a) and hippocampal (b) areas. The MT1/2 mRNAs expression was normalized to that of β-actin mRNA and converted in fold change, compared with the 2-month-old rats. The data represent the mean ± SEM (eight rats per group). The significance of differences is shown. *P < 0.05; aSignificance vs. 2 months; aaSignificance vs. 4 and 8 months.
4 Discussion
Aging is a dominant risk factor for many CNS disorders, among which AD and PD [23,24,26,29]. Aging contributes to the physiological decline of cells and tissues; in the brain, it is associated with inflammation, increased oxidative damage, protein aggregate accumulation and demyelination, whereas it gives rise to a modest neuron and synapse loss [30,31]. Age-related changes in CNS gene expression levels have been reported and many genes, and relative proteins, have been suggested as prognostic biomarkers of neurodegenerative disorders [32–34]. Recently, MT genes have been introduced in the list of early biomarkers of neuronal disorders [27,29]. It has been demonstrated that MTs overexpression in mouse brain provide neuroprotection against deleterious consequences of oxidative and nitrosative stress. However, the authors did not identify the MT isoform(s) responsive to neurodegeneration [27]. Therefore, a major aim of this study was to show whether changes in MT isoforms ratio occur with aging in rats’ hippocampus and cerebral cortex.
MT3 is reported as the most abundant isoform expressed in the brain, and our data are consistent with this result. Here, we also show that the relative abundance of all types of MT transcripts change during aging in both hippocampus and cortex; surprisingly, the first effect is a generalized decrease in the content of MTs transcripts from 2-month-old to 8-month-old rats. This pattern can be explained according to two hypotheses. In the first one, it is possible to assume that the rate of MT expression measured in adolescent rats is strictly linked to the physiological state of the brain, consisting of active, proliferating cells with a high metabolic rate. On the other hand, it cannot be excluded that the higher content of MTs detected in adolescent rats compared with adults is due to the stress that these animals undergo in their early stages of life. Indeed, once in the animal facility, the rats are maintained in individual cages under optimum conditions of humidity, temperature, photoperiod, with water ad libitum and right amount of food; these conditions would lead to a decrease in MTs expression, lacking the stressful factors that may cause their temporary increase.
After passing middle age, at 16 months, we observe a huge increase in MT3 transcripts in both cortical and hippocampal areas, while the MT1/2 mRNA content increases slightly, returning to the levels measured in young rats (2 months). These findings demonstrate an age-related expression of the MT3 gene, whereas the lower levels of MT1/2 genes might suggest a marginal role of these players, if any, in the aging process of the brain. Structural and functional studies on MT3 demonstrated the stronger Cu-thionein character of MT3 compared to that of MT1 and MT2 [35,36], corroborating the hypothesized role of this MT isoform in the brain as a key element in the homeostasis and metabolism of copper ions in the CNS [36]. Recently, Fu et al. have ascertained an age-dependent increase in the Cu levels in rat brains [37].
Therefore, it is conceivable that the increasing amount of MT3 expression in the aging brain might be linked to its different metal-binding behaviour with respect to the other MT isoforms and to its specific neuronal growth inhibitory bioactivity. Together, these features could better meet the needs of aged brain cells.
Disclosure of interest
The authors declare that they have no competing interest.


