1 Introduction
In the last years, interest in parasitological studies on wild fish populations is increasing with the growing importance of fish cultures as a source of protein to human nutrition [1,2]. In the Amazon region of Peru, fish farming represents an activity that is developing rapidly and great interest has focused on several members of the family Cichlidae Heckel, 1840. The interest is attributable primarily to the economic importance of these species, its adaptability to diverse culture systems, good resistance to diseases, excellent feed conversion ratio, and high reproductive capacity [3]. Indeed, cichlids are commercially important for both wild fisheries and aquaculture in Amazonian countries, with the Cichla monoculus Agassiz, 1831 and Chaetobranchus semifasciatus Steindachner, 1875, being a significant example.
Copepods have a wide distribution around the World playing diverse roles in the aquatic environment and infecting farmed and wild fishes of commercial importance [1–6]. In aquaculture, some are beneficial and others are extremely adverse, which may result in entire production losses [1]. Among the species of copepods parasites of fish, Ergasilidae family includes species with high pathogenic potential, some of them responsible for great mortality among cultured fishes in freshwater and brackish environments [1,7]. However, little is known about of the ergasilids which infect cichlids in culturing systems and beyond in natural environment from Peruvian Amazon. In this sense, the present study aimed to evaluate the occurrence of copepods ergasilid in specimens of C. monoculus and C. semifasciatus, two South American freshwater fish of considerable economic importance.
2 Materials and methods
Eighty-five specimens of C. monoculus (8.4 to 22.3 cm in length) and thirty specimens of C. semifasciatus (6.5 to 12.3 cm in length) were collected from of a semi-intensive fish farm, near the city of Nauta (4°30′30′′S, 73°35′00′′W), Loreto State, Peru. The fish were monitored for three months (June to August 2016), during the dry season in the Amazon region. Immediately after collection, the fish were placed in plastic bags containing water, under conditions of artificial aeration, and transported alive to the field laboratory, where they were measured and euthanized by neural pithing, this method was approved by Ethics Committee of the Federal University of São Paulo-UNIFESP (CEUA No. 9209080214), in accordance with Brazilian law (Federal law No. 11.794, dated 8 October 2008 and Federal Decree No. 6899, dated 15 July 2009). All organs were examined using a stereoscopic microscope for parasites infestation. The parasites were removed with dissecting needles from the gill filaments and fixed in 70% ethanol. Small samples of gill tissue infected with adult copepods were removed and examined by means of differential interference contrast (DIC) microscopy at Department of Biophysics, Federal University of São Paulo.
The identification of the parasites was based on the methodology of Araujo and Varella [8] and Thatcher [2]. Holotype and paratypes from Collection of Invertebrates of Instituto Nacional de Pesquisas da Amazônia, Manaus, Brazil (INPA-CR No. 529, female; INPA-CR No. 530a-e, 5 females) were employed in order to support identification. The prevalence and mean intensity of the parasites were calculated according to Bush et al. [9]. The effect of host size on occurrence of copepods was analyzed by Chi2 test. All results were considered significant for P ≤ 0.05.
The physicochemical parameters of the water were measured two times daily (at 8 am and 4 pm) with daily checks of dissolved oxygen, pH, temperature and conductivity by means of a YSI multiparameter meter (Model MPS 556). Ammonium values, hardness, carbon dioxide and total alkalinity were monitored weekly and in the morning (8 am), using a complete package for analysis of freshwater (LaMotte AQ-2).
3 Results
In the present study, 44 (51.7%) C. monoculus and eight (26.6%) C. semifasciatus had adult parasites of ergasilid in their gill filaments. The mean intensity was three and two adult copepods per fish to C. monoculus and C. semifasciatus, respectively. These were not found in any other organs and no clinical signs were observed in the parasitized organ.
Based on the morphology of adult parasites observed via light microscopy, showing body with sub-triangular cephalon, smooth carapace, abdomen with three segments, uropod with two elongated caudal filaments, antennule with six articles, one segment in the exopod of the fourth leg and one serrate spine at the union of the third and fourth segments of the antenna (Fig. 1A-E), the analysis evidenced that these parasites belong to the genus Ergasilus Nordmann, 1832 and the same were identified as Ergasilus coatiarus Araujo and Varella, 1998. From the examined samples, it was evidenced that the host size of the animals did not affect the occurrence of E. coatiarus (χ2 = 0.59, df = 2, P = 0.744). However, to C. monoculus higher occurrence was found in fishes from 18.1 to 22.3 cm long.
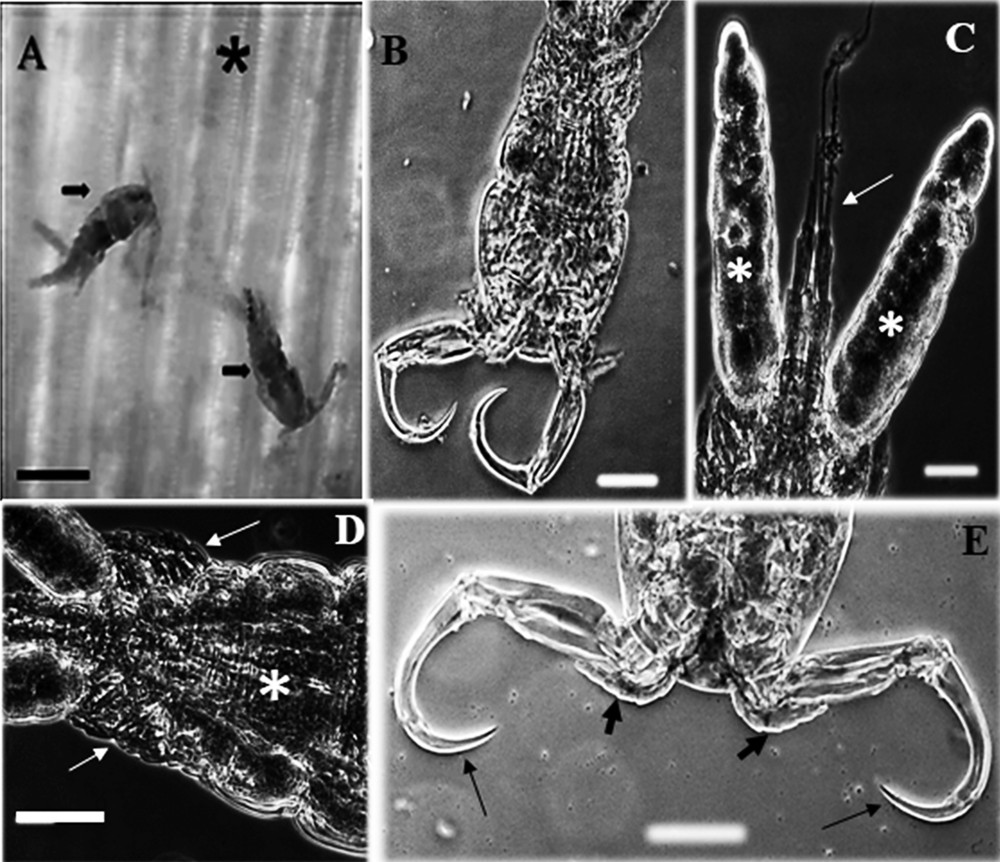
(A-E) Light photomicrographs of Ergasilus coatiarus parasitizing gill filaments of the fish Cichla monoculus and Chaetobranchus semifasciatus. (A) Gills (asterisk) showing adult female of E. coatiarus (arrows). Scale bar = 20 μm. (B) Ventral view of E. coatiarus total. Scale bar = 10 μm. (C) Egg sac (white asterisks) and caudal rami (thin arrow). Scale bar = 10 μm. (D) Ventral view of urosome (asterisk) showing the legs (thin arrows). Scale bar = 10 μm. (E) Ventral view of antennule (large arrows) and antenna (thin arrows). Scale bar = 10 μm.
The values of physicochemical parameters of water in the culture pond were: dissolved oxygen (5.64 ± 0.4 mgL−1), pH (4.83 ± 0.10), temperature (27.23 ± 0.50 °C) and conductivity (106.1 ± 14.0 μScm−1). Ammonium values (0.02 ± 0.10 mgL−1), hardness (21.40 ± 1.80 mgL−1), carbon dioxide (3.2 ± 0.9 mgL−1), and total alkalinity (16.14 ± 0.80 mgL−1).
4 Discussion
Previous studies in Peru have shown widespread distribution of ectoparasites in many wild and cultivated fishes [14], bearing high economic importance in the Amazon region [7,10–13,15]. However, to our knowledge, this study reports for the first time the presence of E. coatiarus in C. semifasciatus in the Amazon basin and is besides the first report of C. monoculus from Peru. The information on morphological data in the present study corroborates the characteristics defined by Araujo and Varella [8] for this species. In the same manner, the site of infection where these parasites were found is in accordance with Araujo and Varella [8], Araujo et al. [16] and Thatcher [2], who reported that specimens of E. coatiarus preferably infect gills of their hosts.
The high occurrence of adult copepods of E. coatiarus in C. monoculus indicates that the infestation by this pathogen is probably frequent during the dry season in the earth ponds. Surely, seasonal occurrence survey is necessary in order to establish the pattern of infection for this parasite, taking in account that seasonal changes represent a combination of many factors influencing the success of a parasite to penetrate a host [17]. In the same way, according Tavares et al. [18], seasonal variation in rainfall levels is a major environmental factor affecting the dynamics of parasite communities in the Amazon region. Nevertheless, there are yet limited number of studies about the effects of the season in infestation patterns of copepods parasite in fish species in the Amazon region, making it difficult to confirm the influence of seasonality on prevalence of these ectoparasites. In this context, future studies to determine the infestation patters by parasites in several wild and cultured fish from Amazon region would be necessary to establish prophylactic strategies to decrease the infestation by ectoparasites, especially when present in farmed fish.
Regarding host specificity, the major ergasilids species show no specificity to parasitize a single host. Indeed, E. coatiarus was also reported parasiting Cichla orinocensis Humboldt, 1821 and Cichla temensis Humboldt, 1821 [16,18], evidencing a low host-specificity of this parasite. In the present study, we reported C. semifasciatus with a new host record to E. coatiarus, despite that low prevalence was observed in the specimens that were examined. However, according to Tavares-Dias et al. [18], E. coatiarus has preference to parasitize fish of the family Cichlidae. This remark may be more related to the consideration that some parasites species prefer hosts with a similar behavior, habitats, additionally to ecological factors [19,20].
In our study, despite the high occurrence of E. coatiarus, no apparent lesions were observed in the fishes. A possible reason may be due to the fact that fishes have shown low level of parasitism. These findings are similar to those of Araujo and Varella [8] and Araujo et al. [16] who reported no disease symptoms in C. monoculus parasitized by E. coatiarus. However, it is well-known that when ectoparasites, in particular copepods and monogeneans species, are present in sufficient number in the gill, they may cause tissue damage and obstruct blood flow, thereby compromising respiratory capacity [1,7,21], taking in account that the gill is the major respiratory organ and plays an important role in nitrogenous waste excretion and in the ionic balance [22].
The results of the present study show that there is not a relationship between the prevalence of parasites and size of examined fish. This finding is in agreement with those described by Ferrari-Hoeinghaus et al. [23], who reported no influence of host size on the prevalence and intensity of parasitism by Amphithecium sp., and Notozothecium sp. in Astyanax altiparanae Garutti and Britski, 2000 and Urocleidoides mastigatus Suriano, 1986 and Scleroductus sp. in Rhamdia quelen Quoy and Gaimard, 1824, but contrast with Siddiqui et al. [24] and Saha et al. [25], who reported a correlation between host size with prevalence and intensity of protozoan parasites in ornamental fish from India.
Although, in our study all water parameters remained within acceptable values for cultivation of tropical fish [26], a moderate prevalence of E. coatiarus was observed in the fish that were examined. In this context, using natural controls to prevent the spread of ectoparasites in fish culture become important, in order to promote avoidance of highly toxic products that could kill or leave the host fish unfeasible for human consumption [7,27]. For instance, Barker and Cone [27] suggest that flow rates above 5 cm/s should impair the transmission of Ergasilus celestis Mueller, 1937 and Pseudodactylogryrus anguillae Yin and Sproston, 1948 in eel aquaculture. Hence, the first recommendation should be the establishment of a water circulation system and manipulation of flow rates in the ponds culture of C. monoculus and C. semifasciatus, preventing the transmission of free-swimming stage of ergasilids and other ectoparasites.
Likewise, the use of temperature and pH manipulation in order to reduce ergasilids infestation could be another recommendation to control these parasites in this aquaculture facility, considering that rates of oviposition and egg hatching of Ergasilus spp. are greater above 23 °C [28]. However, despite that the use of temperature to control fish parasites has had much success with controlling of infections for some taxa of parasites [28,29], manipulation of these abiotic parameters in fish farms from the Amazon region, must take into account the tolerance and sensitivity of fish species that are cultivated. Another recommendation would be continuous exchange of the water to promote elimination of large accumulation of organic matter on the pond bottom, preventing hypoxia and anoxia, which lead the fish more susceptible not only to ectoparasites but also to opportunistic bacteria [30]. Nevertheless, the manipulation of physical parameters in the environment on fish farms of other geographic regions had shown to be effective in order to minimize the transmission of parasites. In the Amazon region, information about this method is still scarce. In this context, future studies about the influence of some abiotic factors on levels of parasitism of gill parasites are highly recommended to prevent and control spreading of ergasilids and other ectoparasites.
Acknowledgements
The authors are grateful to Célio Magalhães, curator of Coleção de Invertebrados do Instituto Nacional de Pesquisas da Amazônia (INPA), for providing holotype and paratypes to support identification of the parasites. O.M. and A.C.M.F.P. thank Sao Paulo Research Foundation, FAPESP, for research grants (process: 2015/23948-5 and 2016/25919-5).


