1 Introduction
As already outlined in several previous publications [1–5], the family Pseudochactidae Gromov, 1998 most certainly contains the most remarkable scorpions described in the last twenty years. The first species to be discovered was Pseudochactas ovchinnikovi Gromov, 1998, found in an isolated mountainous region of southeastern Uzbekistan and southwestern Tajikistan, in Central Asia [6]. A second genus and species, Troglokhammouanus steineri Lourenço, 2007, was described from karst caves in Laos [1]. Although this species was found inside a cave, its morphological characteristics do not correspond to a totally troglobitic element. This Laotian species reopened the question about the origins and affinities of the Pseudochactidae and led to new biogeographical interpretations [1].
In the following years, scorpions have been prospected in karst cave systems in Vietnam, and several specimens of a new pseudochactid scorpion were collected in the Tien Son cave, which belongs to the Phong Nha system. These were described as a new genus and species, Vietbocap canhi Lourenço & Pham, 2010, which represents a true troglobitic element [4]. Subsequent surveys in the cave systems of Vietnam have been carried out and another species of pseudochactid scorpion was collected in the Thien Duong cave, which belongs to the Vom cave system. The new species, Vietbocap thienduongensis Lourenço & Pham, 2012 showed features of a totally troglobitic element [5]. Almost simultaneously, one more new species of Vietbocap was collected in a cave in Laos; Vietbocap lao Lourenço, 2012, once again proved to be a true troglobitic element [2]
Only several years after the description of the first species belonging to the genus Troglokhammouanus Lourenço, 2007, a second species was found and described from another cave in Laos [3]. The description of Troglokhammouanus louisanneorum Lourenço, 2017 was based on a single female specimen and the elements of this genus are apparently less common than those of the genus Vietbocap.
The fact that several pseudochactid elements originating from caves within the same major karst system have been found in Laos and Vietnam suggest that this region of Southeast Asia may represent a refuge or centre of endemism for this family.
In recent years, more intense research was concentrate in the Thien Duong cave, which is a major cave in this karst system (see next section). Prospections were carried on much deeper distances from the entrance of the cave and further elements belonging to the genus Vietbocap were located. The study of the specimens collected respectively at 3000 and 5000 m from the cave entrance showed that these were new species; similar to V. thienduongensis, but presenting some clear morphological differences. This observed situation could suggest a possible case of speciation within the cave system, the first one ever reported for scorpions. Moreover, the population found at 5000 m from the entrance of the cave represent a new record of distance from a cave entrance for scorpions.
We will not re-discuss here the controversial phylogenetic and biogeographical aspects concerning this peculiar scorpion family, since most basic points have already been largely discussed by Lourenço [1]. Using molecular tools, Sharma et al. [7] strongly supported the monophyly of pseudochactids, chaerilids, and buthids. The precise relationships among these three families remain however strongly ambiguous. More detailed information on the orogeny and geodynamics of South East Asia, and on the location, ecology and climate of the national park and caves, can be found in Lourenço and Pham [4].
2 The Thien Duong cave in the Vom cave system
The Thien Duong cave (also called Paradise cave) where the new species were found is located in the Phong Nha–Ke Bang National Park, 60 km northwest of Dông Hói city (Figs. 1–2). The Thien Duong cave is at an elevation of 200 m above sea level, near the west branch of Ho Chi Minh Highway, in Son Trach Commune, Bo Trach District, Quang Binh Province, Vietnam. The cave was discovered by a local people in 2005, and initially the first 5 km of this cave were explored by scientists from the British Cave Research Association in 2005. More recently, the whole extension of the cave was explored by the same Association. The cave is 31 km long, and in parts can reach 100 m in height and 150 m in width. There are two cave systems in Phong Nha Ke Bang region: Phong Nha cave system and Vom cave system. However, these systems are globally isolated, with no geological connections being known between them [8].

The Karstic Massif where the Thien Duong cave is located, showing typical vegetation (photo by Cao Xuan Loc).

Interior view of the Thien Duong cave, 3000 m from the entrance, showing two of the co-authors (T.-H.T. & T.-H. T.) searching for scorpions.
The Phong Nha–Ke Bang karst is the oldest major karst area in Asia. It has been subject to massive tectonic changes and comprises a series of rock types that are interbedded in complex ways. Probably as many as seven major levels of karst development have occurred as a result of tectonic uplift and changing sea levels, thus the karst landscape of PNKB is extremely complex with high geodiversity and many geomorphic features of considerable significance [4,8,9].
3 Methods
Scorpions were collected by scientists of the IEBR and the Phong Nha–Ke Bang National Park, while exploring the cave with the help of standard electric torches. They were found on the cave walls and sometimes under rocks, approximately at 750, 3000 and 5000 m from the main cave entrance. This last distance is a new distance record from a cave entrance for a scorpion. Measurements and illustrations were made using a Wild M5 stereo-microscope with a drawing tube and an ocular micrometer. Measurements follow those of Stahnke [10] and are given in mm. Trichobothrial notations are those developed by Soleglad & Fet [11] and the morphological terminology mostly follows that of Hjelle [12] and Lourenço [1,13].
4 Taxonomic treatment
Family Pseudochactidae Gromov, 1998
Subfamily Vietbocapinae Lourenço, 2012
Genus Vietbocap Lourenço & Pham, 2010
Vietbocap thienduongensis Lourenço & Pham, 2012 (Figs. 3–5)
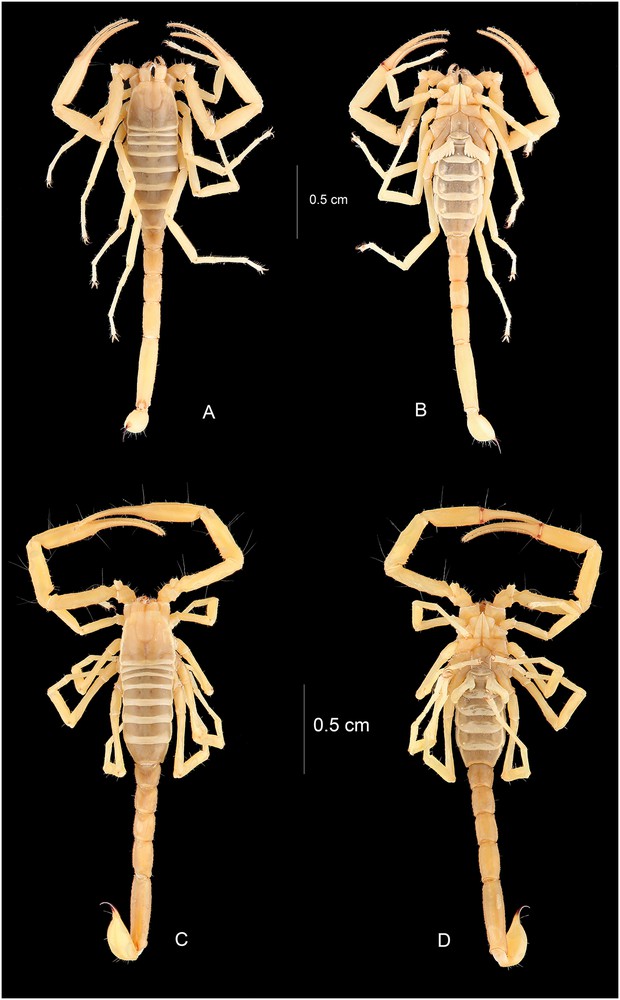
Vietbocap thienduongensis. Habitus, dorsal and ventral aspects. A–B. Male holotype. C–D. Female.

Vietbocap thienduongensis. Male in natural habitat; 800 m from the cave entrance.
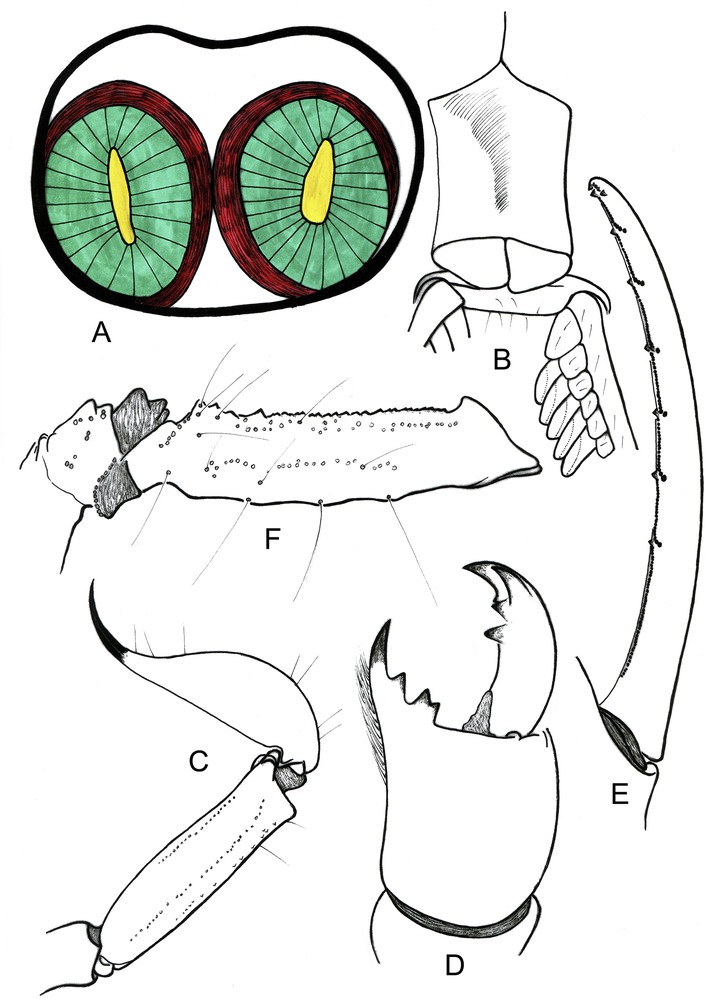
Vietbocap thienduongensis. Female. A. Simple venom glands. B. Sternum, genital operculum and pectines. C. Metasomal segment V and telson, lateral aspect. D. Chelicera, dorsal aspect. E. Cutting edge of movable finger showing rows of granules. F. Femur, dorsal aspect, showing trichobothrial pattern.
Enlarged diagnosis for the female
Vietnam, Quang Binh Province, Phong Nha–Ke National Park, Thien Duong cave (17°31’10.3” N–106°13’22.9”E), initial section of the cave (750 m from the cave entrance), 18/V/2016 (T.-D Do, T.-H. Tran & T.-N. Nguyen), 2 females. Material deposited in the ‘Muséum national d’histoire naturelle’, Paris.
General coloration yellow, less pale than on male for metasomal segments and telson; cheliceral teeth, telson tip, and rows of granules on pedipalp fingers dark reddish. Anterior margin of carapace only slightly depressed, with a concavity slightly stronger than that of male; carapace smooth, except for some isolate granules. Lateral ocelli absent. Pair of circumocular sutures complete in the posterior region to median ocular tubercle with a broad U-shaped configuration. Median ocelli absent; median tubercle represented by a smooth but not depressed zone. Anterosubmedial carinae absent from zone delimited by circumocular sutures; furrows obsolete. Chelicerae shorter in the female; dorsal edge of fixed finger with four denticles (basal, medial, subdistal, distal); ventral edge with 3–4 very reduced denticles; movable finger with three denticles (medial, subdistal, external distal) on dorsal edge, without basal denticles; ventral edge with 4–5 reduced denticles and a weak serrula; external distal denticle smaller than internal distal denticle; ventral aspect of fingers and manus with numerous macrosetae. Type-D trichobothrial pattern [11,14] with 35 trichobothria per pedipalp: 12 on femur, of which 5 dorsal, 4 internal and 3 external (d1, d4, d5 and i4 extremely reduced); 10 on patella, of which 3 dorsal, 1 internal and 6 external (est extremely reduced); ventral surface without trichobothria; 13 trichobothria on chela, of which 5 on manus, 8 on fixed finger (ib2 extremely reduced); dorsal trichobothria of femur with “beta-like” configuration. Sternum pentagonal, type 1 [15], strongly compressed horizontally, slightly longer than wide, external aspect not flat, with a concave region, posteromedian depression round. Pectines each with 3–4 distinct marginal lamellae and 6–7 well-delineated median lamellae; fulcra absent; pectinal tooth count 7–7 and 7–8. Genital operculum completely divided longitudinally. Telotarsi each with several spinular setae, not clearly arranged in rows. Metasomal segment V with a weakly marked pair of ventrosubmedian carinae; no ventromedian carina between ventrosubmedian carinae; metasomal carinae better marked than on male. Pedipalps shorter than those of male; fixed and movable fingers strongly curved, but less than on male; dentate margins each with median denticle row comprising seven oblique granular sub-rows; internal and external accessory granules at base of each sub-row. Respiratory spiracles small, semi-oval to round. Pro- and retrolateral pedal spurs present on legs I–IV. Tibial spurs absent from all legs. Telson long and less bulbous than on male; vesicle smooth on all faces; aculeus shorter than vesicle and weakly curved without a subaculear tubercle ventrally. Form of venom glands extremely simples with a total absence of folds (Fig. 5).
Measurements (in mm) of female Vietbocap thienduongensis
Total length 23.9. Carapace: length 3.0; anterior width 2.0; posterior width 3.2. Mesosoma length 5.5. Metasomal segments: I, length 1.2, width 1.5; II, length 1.4, width 1.2; III, length 1.7, width 1.1; IV, length 2.2, width 1.1; V, length 4.3, width 1.0, depth 1.0. Telson length 4.6; vesicle width 1.4, depth 1.2. Pedipalp: femur length 4.0, width 1.0; patella length 3.8, width 1.2; chela length 7.5, width 1.2, depth 1.2; movable finger length 4.4.
Reports: Sternum length/width, 1.2/1.2 = 1.00. Chela length/movable finger length, 7.5/4.4 = 1.70.
Vietbocap aurantiacus sp. n. (Figs. 6–7)
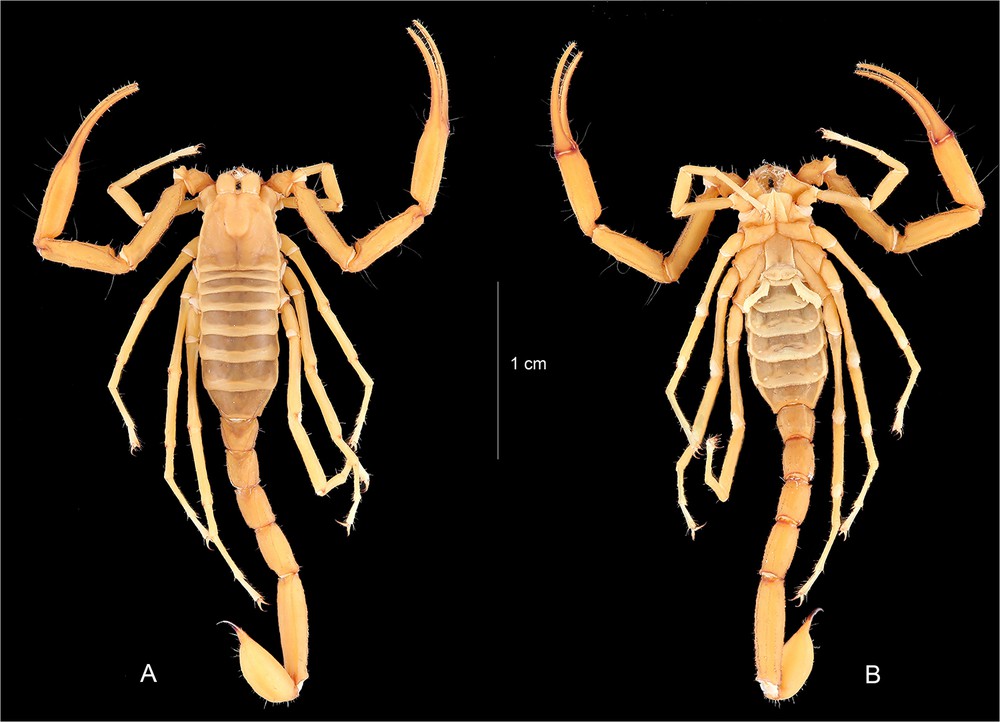
Vietbocap aurantiacus sp. n. Female holotype. Habitus, dorsal and ventral aspects.
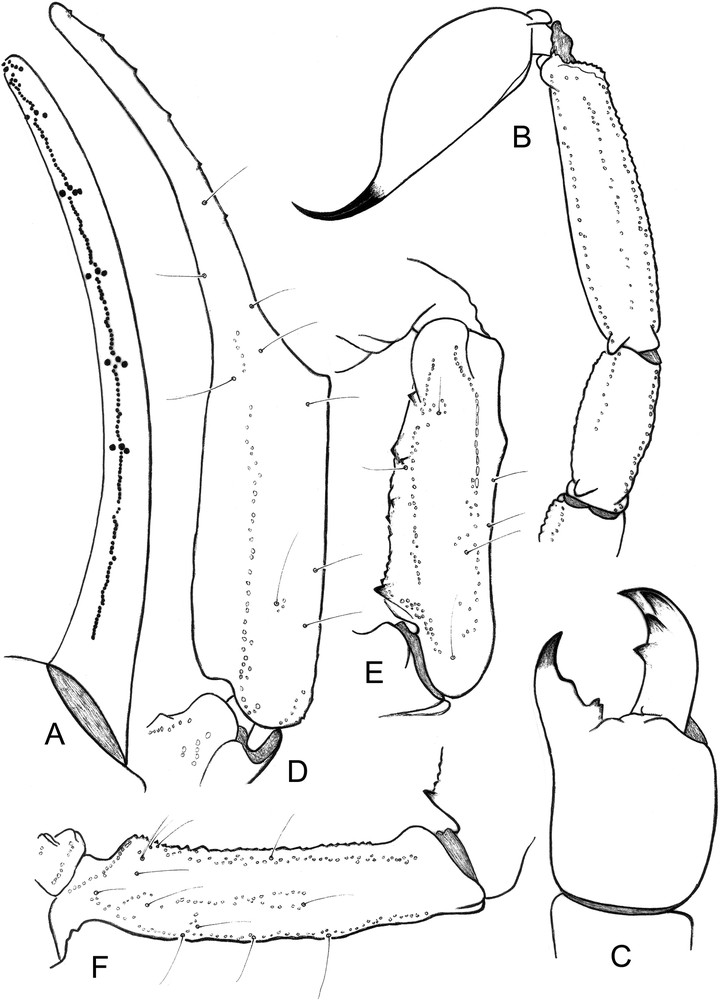
Vietbocap aurantiacus sp. n. Female holotype. A. Cutting edge of movable finger showing rows of granules. B. Metasomal segments IV–V and telson, lateral aspect. C. Chelicera, dorsal aspect. D–F. Trichobothrial pattern. D. Chela, dorso-external aspect. E–F. Patella and femur, dorsal aspect.
Diagnosis: Anterior margin of carapace not depressed with a weak to moderate concavity. Lateral ocelli absent. Pair of circumocular sutures complete in the posterior region to median ocular tubercle with a broad U-shaped configuration. Median ocelli absent; median tubercle represented by a smooth but not depressed zone. Anterosubmedial carinae absent from zone delimited by circumocular sutures. Type-D trichobothrial pattern [11,14] with 35 trichobothria per pedipalp: 12 on femur, of which 5 dorsal, 4 internal and 3 external (d1, d4, d5 and i4 extremely reduced); 10 on patella, of which 3 dorsal, 1 internal and 6 external (est extremely reduced); ventral surface without trichobothria; 13 trichobothria on chela, of which 5 on manus, 8 on fixed finger (ib2 extremely reduced); dorsal trichobothria of femur with “beta-like” configuration. Sternum pentagonal, type 1 [15], strongly compressed horizontally, longer than wide, external aspect not flat, with a concave region, posteromedian depression round Sternite V with a white posterior inflated triangular zone. Telotarsi each with several spinular setae, not clearly arranged in rows. Metasomal segment V with a moderately marked pair of ventrosubmedian carinae; no ventromedian carina between ventrosubmedian carinae. Fixed and movable fingers strongly curved; dentate margins each with median denticle row comprising seven oblique granular sub-rows; internal and external accessory granules at base of each sub-row. Carinae on metasoma and pedipalps better marked than on the other species. Respiratory spiracles small, semi-oval to round. Pro- and retrolateral pedal spurs present on legs I-IV. Tibial spurs absent from all legs.
Type material: Female holotype, two female paratypes. Vietnam, Quang Binh Province, Phong Nha–Ke Bang National Park, Thien Duong cave (17°31′10.3″N–106°13′22.9″E), mid-section of cave (3000 m from cave entrance), 23/V/2013 (D.-S. Pham). Holotype and one paratype deposited in the “Muséum national d’histoire naturelle”, Paris. One paratype deposited in the Institute of Ecology and Biological Resources, Vietnam Academy of Science and Technology, Hanoi.
Etymology. The specific name is a Latin adjective referring to the orange coloration of the new species (aurantiacus in Latin).
Description based on holotype and paratypes (measurements given after the description).
Colour. General coloration yellow to reddish-yellow, darker than in the other species of the genus; cheliceral teeth, telson tip, pedipalpal and metasomal carinae and rows of granules on pedipalp fingers dark reddish.
Morphology. Chelicerae: dorsal edge of fixed finger with four denticles (basal, medial, subdistal, distal); ventral edge with 3–4 very reduced denticles; movable finger with three denticles (medial, subdistal, external distal) on dorsal edge, without basal denticles; ventral edge with 4–5 reduced denticles and a moderate serrula; external distal denticle smaller than internal distal denticle; ventral aspect of fingers and manus with numerous macrosetae. Carapace: anterior margin not depressed with a weakly to moderately marked concavity; lateral ocelli absent; median ocular tubercle represented by a smooth and not depressed zone; median ocelli absent; interocular furrow obsolete. One pair of weakly marked circumocular sutures with a broad U-shaped configuration, also complete behind median ocular tubercle. Anteromedian and posteromedian furrows shallow; posterolateral furrow shallow, weakly curved; posteromarginal furrow narrow, shallow. Carapace almost totally smooth, except for some isolated granules anteriorly. Pedipalp segments apilose. Femur with five strongly marked carinae; intercarinal surfaces smooth. Patella with six strongly marked carinae; ventrointernal carinae with some spinoid granules; intercarinal surfaces smooth. Chela with dorso-external and ventral carinae moderately marked; tegument smooth. Fixed and movable fingers strongly curved; dentate margins, each with median denticle row comprising seven oblique granular sub-rows; each sub-row comprising several small granules and internal and external accessory granules. Trichobothria orthobothriotaxic, Type D [11,14], “beta-like” configuration, d2 situated on dorsal surface, d3 and d4 in same axis of the femur, parallel and closer to dorsoexternal carina than is d1, angle formed by d1, d3 and d4 opening toward internal surface; totals: femur, 12 (5 dorsal, 4 internal, 3 external); patella, 10 (3 dorsal, 1 internal, 6 external); chela, 13 (5 on manus, 8 on fixed finger). Legs I to IV: tibiae without spurs; basitarsi each with a pair of pro- and retrolateral spurs; telotarsi each with several spinular setae, not clearly arranged in rows. Sternum pentagonal, type 1 [15], strongly compressed horizontally, longer than wide, external aspect not flat, with a concave region, posteromedian depression round. Pectines each with 3–4 distinct marginal lamellae and 7–8 well-delineated median lamellae in females. Fulcra absent. Pectinal tooth count: 7–7 in the female holotype, 6–7, 6–6 in female paratypes. Genital operculum completely divided longitudinally. Mesosoma: pre-tergites smooth and shiny; post-tergites II–VI smooth, without granules; VII with a few granules and a pair of dorso-submedian and dorsolateral carinae, reaching posterior edge of segment. Sternites entirely smooth, acarinate; V with a white posterior inflated triangular zone; surfaces with scattered macrosetae; distal margins with sparse row of macrosetae; respiratory spiracles small, semi-oval to round. Metasoma with a few short macrosetae. Ten carinae on segments I to III, weakly marked on II–III; eight carinae on segment IV; four on segment V. Dorso-submedian carinae moderately developed on segments I–IV, absent on segment V; spinoid granules absent. Other carinae moderately to weakly developed on segments I–V. Telson long and slightly bulbous; vesicle smooth on all faces; aculeus shorter than vesicle and weakly curved, without a subaculear tubercle ventrally. Form of venom glands unknown, but most certainly similar to that of V. thienduongensis.
Measurements (in mm) of female holotype of Vietbocap aurantiacus sp. n.
Total length 35.8. Carapace: length 4.5; anterior width 2.7; posterior width 4.8. Mesosoma length 8.4. Metasomal segments: I, length 1.9, width 2.2; II, length 2.2, width 1.9; III, length 2.5, width 1.8; IV, length 3.2, width 1.7; V, length 6.3, width 1.6, depth 1.4. Telson length 6.8; vesicle width 2.2, depth 1.9. Pedipalp: femur length 6.0, width 1.3; patella length 5.5, width 1.6; chela length 10.6, width 1.7, depth 1.6; movable finger length 6.3.
Ratios: sternum length/width, 2.1/1.8 = 1.67; chela length/movable finger length, 10.6/6.3 = 1.68.
Relationships
It is noticeable to recall that the material used in the description of Vietbocap aurantiacus sp. n. was available for study since a number of years now; however, only females of this species were available for the study. Contrarily, Vietbocap thienduongensis was described on the base of males only. Consequently, an objective comparative analysis of both species becomes possible only after the more recent discovery of females of V. thienduongensis (see previous section). The general morphologies of all known species of Vietbocap are particularly similar. Therefore, the identification of these very closely related species can only be based on some rather discrete features.
Vietbocap thienduongensis and Vietbocap aurantiacus sp. n. are the two most geographically close species found in the cave, distant of 1.0 to 1.5 km. V. auranticus sp. n. can, however, be distinguished by a number of features:
- • bigger size (35.8 vs 23.9 mm) and distinct morphometric values (see measurements and ratios following the description);
- • darker coloration, more to orange-yellow;
- • anterior margin of carapace not depressed;
- • sternum longer than wide (see ratios following the description);
- • metasomal segments and pedipalps better carinated and granulated;
- • sternite V with a conspicuous white posterior inflated triangular zone;
- • moderate serrula on chelicera movable finger.
Vietbocap quinquemilia sp. n. (Figs. 8–11)
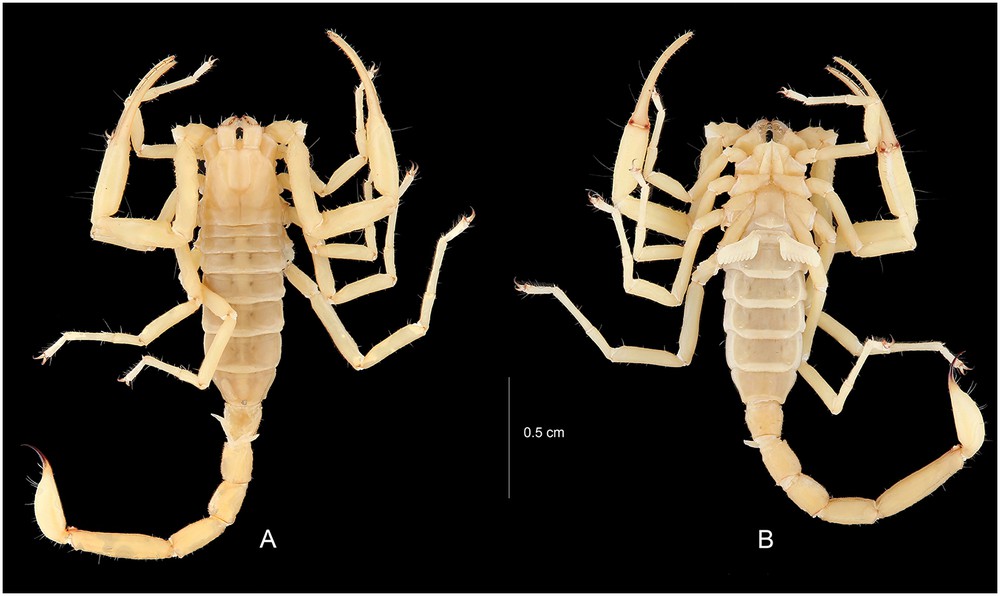
Vietbocap quinquemilia sp. n. Male holotype. Habitus, dorsal and ventral aspects.

Vietbocap quinquemilia sp. n. Male in natural habitat; 5000 m from cave entrance.
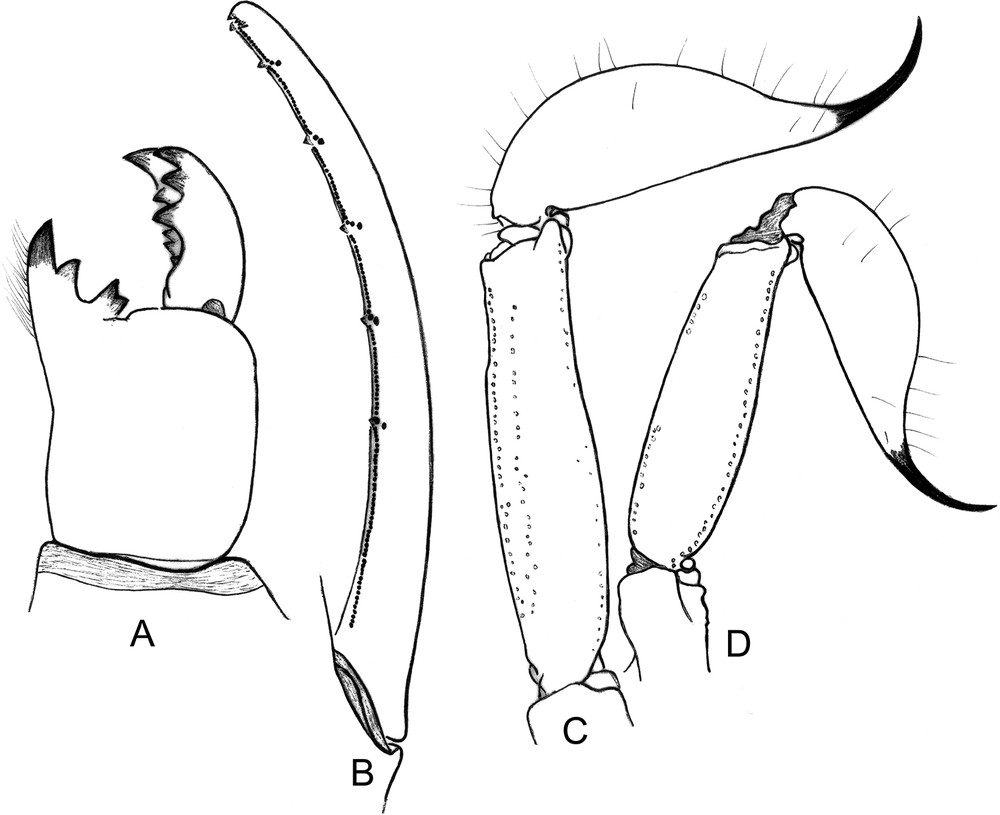
Vietbocap quinquemilia sp. n. A–C. Male holotype. D. Female paratype. A. Chelicera, dorsal aspect. B. Cutting edge of movable finger showing rows of granules. C–D. Metasomal segment V and telson, lateral aspect.
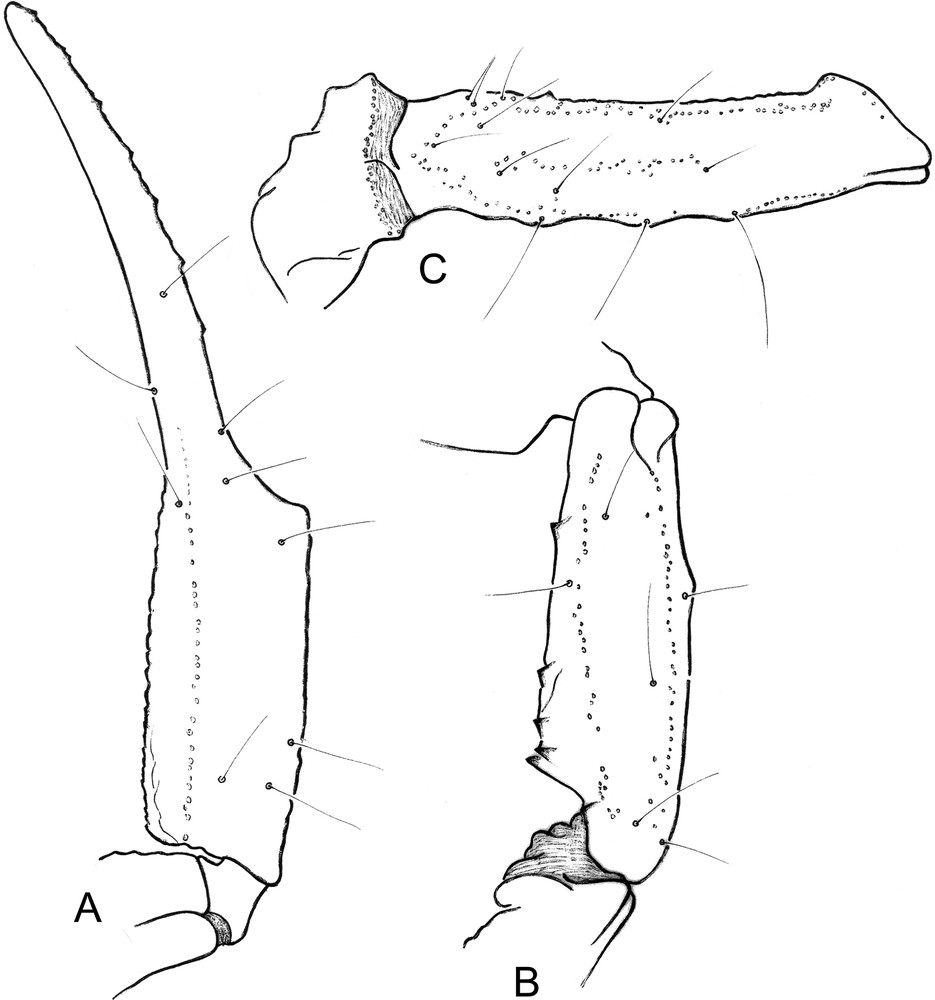
Vietbocap quinquemilia sp. n. Male holotype. A–C. Trichobothrial pattern. A. Chela, dorso-external aspect. B–C. Patella and femur, dorsal aspect.
Diagnosis: anterior margin of carapace slightly depressed, with a weak concavity. Lateral ocelli absent. Pair of circumocular sutures complete in the posterior region to median ocular tubercle with a broad U-shaped configuration. Median ocelli absent; median tubercle represented by a smooth and only slightly depressed zone. Anterosubmedial carinae absent from zone delimited by circumocular sutures. Type-D trichobothrial pattern [11,14] with 35 trichobothria per pedipalp: 12 on femur, of which 5 dorsal, 4 internal and 3 external (d1, d4, d5 and i4 extremely reduced); 10 on patella, of which 3 dorsal, 1 internal and 6 external (est extremely reduced); ventral surface without trichobothria; 13 trichobothria on chela, of which 5 on manus, 8 on fixed finger (ib2 extremely reduced); dorsal trichobothria of femur with “beta-like” configuration. Sternum pentagonal, type 1 [15], strongly compressed horizontally, slightly longer than wide, external aspect not flat, with a concave region, posteromedian depression round. Telotarsi each with several spinular setae, not clearly arranged in rows. Metasomal segment V with a weakly marked pair of ventrosubmedian carinae; no ventromedian carina between ventrosubmedian carinae. Fixed and movable fingers strongly curved; dentate margins each with median denticle row comprising seven oblique granular sub-rows; internal and external accessory granules at base of each sub-row. Respiratory spiracles small, semi-oval to round. Pro- and retrolateral pedal spurs present on legs I–IV. Tibial spurs absent from all legs.
Type material: Male holotype, two female paratypes. Vietnam, Quang Binh Province, Phong Nha - Ke Bang National Park, Thien Duong cave (17°31′10.3″N–106°13′22.9″E), mid-section of cave (5000 m from cave entrance), 6/IV/2015 (D.-S. Pham). Holotype and one paratype deposited in the “Muséum national d’histoire naturelle”, Paris. One paratype deposited in the Institute of Ecology and Biological Resources, Vietnam Academy of Science and Technology, Hanoi.
Etymology. The specific name is a Latin noun in apposition referring to the distance from the cave entrance, 5000 m (quinquemilia in Latin) where the new species was found.
Description based on male holotype and female paratypes (measurements given after the description).
Colour. General coloration very pale yellow almost whitish, paler than all the other known species in the genus; cheliceral teeth, telson tip and rows of granules on pedipalp fingers slightly reddish.
Morphology. Chelicerae: dorsal edge of fixed finger with four denticles (basal, medial, subdistal, distal); ventral edge with 3–4 very reduced denticles; movable finger with three denticles (medial, subdistal, external distal) on the dorsal edge, without basal denticles; ventral edge with 4–5 reduced denticles and a moderate serrula; external distal denticle smaller than internal distal denticle; ventral aspect of fingers and manus with numerous macrosetae. Carapace: anterior margin only slightly depressed with a weakly marked concavity; lateral ocelli absent; median ocular tubercle represented by a smooth and only slightly depressed zone; median ocelli absent; interocular furrow obsolete. One pair of weakly marked circumocular sutures with a broad U-shaped configuration, also complete behind median ocular tubercle. Anteromedian and posteromedian furrows shallow; posterolateral furrow shallow, weakly curved; posteromarginal furrow narrow, shallow. Carapace almost entirely smooth, except for some very isolated granules anteriorly. Pedipalp segments apilose. Femur with five carinae, all moderate to weak; intercarinal surfaces smooth. Patella with six discernible carinae; ventrointernal carinae with some spinoid granules; intercarinal surfaces smooth. Chela with dorso-external and ventral carinae weakly marked; tegument smooth. Fixed and movable fingers strongly curved; dentate margins, each with median denticle row comprising seven oblique granular sub-rows; each sub-row comprising several small granules and internal and external accessory granules. Trichobothria orthobothriotaxic, type D [11,14], “beta-like” configuration, d2 situated on dorsal surface, d3 and d4 in same axis of the femur, parallel and closer to the dorsoexternal carina than is d1, angle formed by d1, d3 and d4 opening toward the internal surface; totals: femur, 12 (5 dorsal, 4 internal, 3 external); patella, 10 (3 dorsal, 1 internal, 6 external); chela, 13 (5 on manus, 8 on fixed finger). Legs I to IV: tibiae without spurs; basitarsi each with a pair of pro- and retrolateral spurs; telotarsi each with several spinular setae, not clearly arranged in rows. Sternum pentagonal, type 1 [15], strongly compressed horizontally, slightly longer than wide, external aspect not flat, with a concave region, posteromedian depression round. Pectines each with 3 distinct marginal lamellae and 7–8 well-delineated median lamellae in both sexes. Fulcra absent. Pectinal tooth count: 8–8 in males, 7–7 in females. Genital operculum completely divided longitudinally; genital plugs observed in the male. Mesosoma: pre-tergites smooth and shiny; post-tergites II–VI smooth; granules totally absent; VII equally without granules and a pair of dorso-submedian and dorsolateral carinae, reaching posterior edge of segment. Sternites almost entirely smooth, acarinate; surfaces with scattered macrosetae; distal margins with sparse row of macrosetae; respiratory spiracles small, semi-oval to round. Metasoma with a few short macrosetae. Ten carinae on segments I to III; eight carinae on segment IV; four on segment V. Dorso-submedian carinae moderately developed on segments I–IV, absent on segment V; spinoid granules absent. Other carinae moderately to weakly developed on segments I–V. Telson long and slightly bulbous in the male, slender in the female; vesicle smooth on all faces; aculeus shorter than vesicle and weakly curved, without a subaculear tubercle ventrally. Form of venom glands unknown, but most certainly similar to that of V. thienduongensis.
Measurements (in mm) of male holotype and female paratype of Vietbocap quinquemilia sp. n.
Total length 25.4/20.2. Carapace: length 3.2/2.5; anterior width 2.0/1.8; posterior width 3.3/2.7. Mesosoma length 7.4/6.1. Metasomal segments: I, length 1.2/1.0, width 1.6/1.2; II, length 1.4/1.2, width 1.4/1.0; III, length 1.6/1.3, width 1.3/0.9; IV, length 2.1/1.6, width 1.2/0.8; V, length 4.2/3.2, width 1.2/0.8, depth 1.1/0.8. Telson length 4.3/3.3; vesicle width 1.4/1.1, depth 1.2/0.9. Pedipalp: femur length 4.1/2.9, width 1.0/0.8; patella length 4.0/3.1, width 1.1/0.9; chela length 7.5/5.7, width 1.2/1.0, depth 1.1/0.9; movable finger length 4.4/3.4.
Reports: Male: Sternum length/width, 1.4/1.2 = 1.17. Chela length/movable finger length, 7.5/4.4 = 1.70. Female: Sternum length/width, 1.1/1.0 = 1.10. Chela length/movable finger length, 5.7/3.4 = 1.68.
Relationships
Vietbocap quinquemilia sp. n. is geographically separated of Vietbocap aurantiacus sp. n. by 2 km. Their general morphology although similar presents a number of differences and in fact V. quinquemilia seems more closely related to V. thienduongensis. This new species can however be characterized by a number of particular features:
- • small size (only 20.2 mm for female) and distinct morphometric values (see measurements and ratios following the description);
- • a very pale coloration almost whitish; this is the paler species known for the genus;
- • median ocular tubercle on carapace only slightly depressed;
- • cheliceral serrula moderately marked;
- • tergites globally smooth;
- • pedipalp carinae very weakly marked.
5 Discussion
Cases of more than one species of a given genus, living in a common cave system, have been recorded for other zoological groups. For example, Bayer and Jäger [16] reported the presence of two spiders of the genus Heteropoda, namely Heteropoda maxima and Heteropoda steineri in the Xe Bangfai Cave system. Although these authors indicate that H. steineri would be more restricted to deeper parts in the cave, they suggest that these two very mobile species could potentially meet. A noticeable parallel case was also observed for the species of the cave scorpion genus Alacran Francke in Mexico [17]. In temperate regions, several species of a same beetle genus are known to co-occur frequently in a same cave, like for the Aphaenops in the Pierre Saint-Martin system in French Pyrenees [18]. Most reported cases however show that these parapatric and/or almost sympatric species present marked distinct degrees of adaptation to cave and/or subterranean life.
The general assumption that cave species evolved, in most cases, directly from surface ancestors, was recently questioned by Juan et al. [19]. Based on phylogeographic studies, these authors confirmed that cave species were often cryptic and presenting highly restricted distributions. These studies also suggested that their divergence and potential speciation processes may occur despite the presence of gene flow from surface populations. Finally, they concluded that these same studies could provide evidence for speciation and adaptive evolution within the confines of cave systems [19].
Vietbocap, as the other genera associated with the family Pseudochactidae, can be considered as one of the most, if not the most basic lineage among known scorpions. If not all authors agree about the precise phylogenetic position of pseudochactids, they do agree about its basal position [7,20]. The Vietbocap “populations” found in the Thien Duong cave show a very similar degree of cave adaptation, with a complete regression of eyes and, in most cases, a very marked regression of the pigmentation. On the other hand, Vietbocap epigean relatives remain unknown. In fact, epigean elements of the family Pseudochactidae are not known from South-East Asia. The original epigean ancestor of the Vietbocap lineage was most certainly an ancient colonizer of underground habitats; however, the totality of the present known species probably did not derived from a surface ancestor. This attests to a rather old process of adaptation to subterranean environments. Although available data about the scorpion populations of the different species are still very limited and phylogenetic evidence are still lacking, it can be considered that the speciation process took place after the colonization of the subterranean habitat by the epigean ancestor of the Vietbocap lineage.
As already exposed, the Thien Duong cave is a huge cave with 31 km of passages, in several sectors 100 m in height and 150 m in width; most present physical parameters inside the cave such as air temperature and humidity can be considered rather similar. Nevertheless, the geological history of the cave system is very old and most certainly knew a number of vicissitudes during geological time, probably causing collapsing situations with the creation of local barriers, maybe long enough in time to allow full processes of speciation. No data is, however, available to confirm any hypothesis in this sense.
It is difficult to estimate from the sole morphological study of these “populations” of Vietbocap living in the Thien Duong cave what is their precise degree of differentiation. The processes of differentiation and speciation in scorpions are probably rather slow, since the group shows long-term reproduction strategies and a low number of generations when compared to other arthropods [21]. Consequently, the question to be addressed is: are we faced with species, subspecies or only morphs of a large polymorphic species? For three of these populations, a specific status is here suggested in association with their possible allopatric distribution, though the number of available specimens is small to evaluate the robustness of the observed differences. In this case, the condition of superspecies sensu Mayr [22] could be suggested to the global Vietbocap lineage. The species within each sub-lineage would be represented by allopatric, parapatric our weakly sympatric groups, really or potentially inter-sterile in natural conditions [23]. Each one of these different species can therefore be defined as a Prospecies in the sense of Birula [24]. The main cue, however, remains the discovery and description of an epigean element associated with Vietbocap, which however probably knew a negative selection during the evolutionary time.
The presence of syntopic and closely related troglobitic species of a same genus in a same cave is quite exceptional. Molecular approaches will be necessary to evaluate the robustness of the observed differences between the species which have been recognized.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgements
We wish to express our thanks to Élise-Anne Leguin (MNHN, Paris) for the preparation of the photos and plates; to Adriano Kury (Museu Nacional, Rio de Janeiro), Louis Deharveng (MNHN, Paris), and Lucienne Wilmé (Missouri Botanical Garden) for their useful comments to the manuscript. The second author is most grateful to the research project of the Vietnam Academy of Science and Technology (VAST04.09/16-17) and to the NAFOSTED grant (106NN.06-2015.38), which financially supported him for field works.


