1 Introduction
One of the pigs endemic to the Indonesian islands of Buru, Sulawesi and a number of the smaller islands in their vicinity is the babirusa (genus Babyrousa). The unusual anatomy of the canine teeth of the adult male has long made this animal the subject of curiosity and scientific investigation [1–5]. Study of its teeth in particular has generated a better understanding of the babirusa's agonistic behaviour [6–8]. The normal growth of the open-rooted maxillary canine teeth from birth to adulthood, as recently described, involves the rotation of the maxillary canine tooth alveolus in a subcutaneous median plane to bring the tooth to point first rostrally and then progressively in a dorsal direction [9]. The tooth tip then grows out of the alveolus and pierces through the skin lateral to the nose, its curled tip pointing caudally. As it elongates the axial curvature is reduced, lifting the tip of each canine tooth along the median plane of the skull, up and over first the nasal bones and then over the frontal bone [9,10]. However, not all maxillary canine teeth follow this normal pattern. Grzimek [11] and Mohr [12] reported a male babirusa in Brookfield zoo, Chicago that had maxillary canine teeth that projected rostrally and crossed one another, left over right. McIntosh [13] illustrated part of a skull where the right maxillary canine had punctured the parietal bone. Awareness of canine maxillary tooth growth under the enhanced dietary regime of zoological collections has for a number of years prompted veterinary caution and the early trimming of canine teeth [14,15]. It was widely suggested on Buru Island in Maluku, Indonesia, recently that the maxillary teeth of the babirusa there eventually grow into the eyes of the male and to cause blindness and death [5].
The incidence of aberrant canine tooth growth in the babirusa has not been studied before. The opportunity to do so was presented by a worldwide survey of babirusa skulls curated in museum and private collections. Of these 431 were adult male and had retained at least one maxillary canine tooth. Evidence of anomalous canine tooth growth was looked for in this sample, categorised in relation to the normal pattern of tooth growth, and quantified. The results have been analysed to identify potential causes of the aberrations found and to suggest methods of amelioration.
2 Materials and methods
Eighty-three adult male babirusa skulls were identified as exhibiting aberrant maxillary canine tooth growth (Table 1). Of these 24 skulls represented babirusa from Buru and the Sula Islands, and 45 skulls represented babirusa from Sulawesi and the Togian Islands. The remaining series of 14 babirusa skulls originally came from zoo animals; based on morphometric criteria [2] and studbook data [17], it was deduced that these originated from Sulawesi Island. An additional five Sulawesi skulls showing mandibular alveolar anomalies were included in this study for discussion purposes (Table 1). The criteria for normal maxillary canine growth of the male babirusa have recently been described [10].
A list of the international reference numbers for the 83 babirusa skulls (AAM number), their Indonesian geographic origin, the museum collection where they are curated, the museum country, their registered museum specimen number and the maxillary canine anomaly noted.
| AAM number | Indonesian region | Museum Collection | Country | Museum ID | Aberrant growth |
| AAM0004 | Sula or Buru Islands | Zoological Museum Amsterdam | Netherlands | ZMA.MAM.00805 | 2 |
| AAM0016 | Sulawesi | Zoological Museum Amsterdam | Netherlands | ZMA.MAM.01184 | 1 |
| AAM0024 | Sulawesi | Zoological Museum Amsterdam | Netherlands | ZMA.MAM.02242 | 5 |
| AAM0025 | Sulawesi | Zool Museum Amsterdam | Netherlands | ZMA.MAM.08936 | 9 |
| AAM0031 | Sula Islands | Zoological Museum Amsterdam | Netherlands | ZMA.MAM.09116 | 2 |
| AAM0050 | Sula or Buru Islands | Zoological Museum Amsterdam | Netherlands | ZMA.MAM.25389 | 1 |
| AAM0059 | Sulawesi | Naturhistorisches Museum Basel | Switzerland | C.2882 | 2 |
| AAM0066 | Sula or Buru Islands | Naturhistorisches Museum Basel | Switzerland | C.6204 | 2 |
| AAM0068 | Sula or Buru Islands | Naturhistorisches Museum Basel | Switzerland | C.111.196 | 2 |
| AAM0080 | Sulawesi | Museum für Naturkunde, Berlin | Germany | 1969 | 9 |
| AAM0084 | Sulawesi | Museum für Naturkunde, Berlin | Germany | 8433 | 8 |
| AAM0085 | Sulawesi | Museum für Naturkunde, Berlin | Germany | 8435 | 2 |
| AAM0086 | Sulawesi | Museum für Naturkunde, Berlin | Germany | 8437_cran | 2 |
| AAM0091 | Sula or Buru Islands | Museum für Naturkunde, Berlin | Germany | 33677 | 4 |
| AAM0099 | Sulawesi | Museum für Naturkunde, Berlin | Germany | 46526 | 2, 5 |
| AAM0104 | Sula or Buru Islands | Museum für Naturkunde, Berlin | Germany | 64534 | 4 |
| AAM0109 | Sula or Buru Islands | Museum für Naturkunde, Berlin | Germany | A2620 | 1 |
| AAM0137 | Buru | Museum Zoologicum Bogoriense, Cibinong | Indonesia | 1081 | 4 |
| AAM0154 | Sulawesi | Museum Zoologicum Bogoriense, Cibinong | Indonesia | 6904 | 8 |
| AAM0158 | Buru | Museum Zoologicum Bogoriense, Cibinong | Indonesia | 6908 | 4 |
| AAM0195 | Sulawesi | Zoologisk Museum, København | Denmark | M0321 | 2 |
| AAM0198 | Sulawesi | Zoologisk Museum, København | Denmark | M1433 | 5 |
| AAM0203 | Sula or Buru Islands | Skolen for Veterinærmedicin og Husdyrvidenskab, Københavns Universitet | Denmark | Vet2 | 2, 4 |
| AAM0222 | Sulawesi | Senckenberg Naturhistorische Sammlungen Dresden | Germany | 3070 | 5 |
| AAM0231 | Sulawesi | National Museum of Scotland, Edinburgh | Scotland | NMS.Z.1878.1.4 | 5 |
| AAM0246 | Zoo | National Museum of Scotland, Edinburgh | Scotland | NMS.Z.1993.159.003 | 1 |
| AAM0252 | Zoo | National Museum of Scotland, Edinburgh | Scotland | NMS.Z.1999.185 | 2 |
| AAM0253 | Zoo | National Museum of Scotland, Edinburgh | Scotland | NMS.Z.1999.282.001 | 1 |
| AAM0267 | Zoo | National Museum of Scotland, Edinburgh | Scotland | NMS.Z.2004.161.002 | 2 |
| AAM0270 | Zoo | National Museum of Scotland, Edinburgh | Scotland | NMS.Z.2006.033.002 | 1 |
| AAM0272 | Zoo | National Museum of Scotland, Edinburgh | Scotland | NMS.Z..2006.082 | 2 |
| AAM0275 | Zoo | National Museum of Scotland, Edinburgh | Scotland | NMS.Z.2008.026. Morvell | 2 |
| AAM0283 | Sulawesi | Naturmuseum Senckenberg, Frankfurt Am Main | Germany | 423 | 2 |
| AAM0287 | Buru | Naturmuseum Senckenberg, Frankfurt Am Main | Germany | 427 | 4 |
| AAM0288 | Buru | Naturmuseum Senckenberg, Frankfurt Am Main | Germany | 429 | 5 |
| AAM0290 | Sulawesi | Naturmuseum Senckenberg, Frankfurt Am Main | Germany | 1588 | 6 |
| AAM0293 | Zoo | Naturmuseum Senckenberg, Frankfurt Am Main | Germany | 5951 | 2 |
| AAM0317 | Sulawesi | Göteborgs naturhistoriska museum, Göteborg | Sweden | 17.938 | 2 |
| AAM0319 | Sulawesi | Göteborgs naturhistoriska museum, Göteborg | Sweden | 17.940 | 2 |
| AAM0323 | Sulawesi | Göteborgs naturhistoriska museum, Göteborg | Sweden | 17.944 | 5 |
| AAM0326 | Sulawesi | Göteborgs naturhistoriska museum, Göteborg | Sweden | 17.947 | 2, 9 |
| AAM0333 | Sulawesi | Göteborgs naturhistoriska museum, Göteborg | Sweden | 17.954 | 5 |
| AAM0334 | Sulawesi | Göteborgs naturhistoriska museum, Göteborg | Sweden | 17.955 | 5 |
| AAM0336 | Sulawesi | Göteborgs naturhistoriska museum, Göteborg | Sweden | 17.957 | 1 |
| AAM0337 | Sulawesi | Göteborgs naturhistoriska museum, Göteborg | Sweden | 17.958 | 6 |
| AAM0339 | Sulawesi | Göteborgs naturhistoriska museum, Göteborg | Sweden | 17.960 | 9 |
| AAM0340 | Sulawesi | Göteborgs naturhistoriska museum, Göteborg | Sweden | 17.961 | 2 |
| AAM0347 | Sulawesi | Göteborgs naturhistoriska museum, Göteborg | Sweden | 17.968 | 2 |
| AAM0357 | Sulawesi | Göteborgs naturhistoriska museum, Göteborg | Sweden | 17.978 | 1, 9 |
| AAM0365 | Sulawesi | Göteborgs naturhistoriska museum, Göteborg | Sweden | 17.986 | 5 |
| AAM0369 | Sulawesi | Göteborgs naturhistoriska museum, Göteborg | Sweden | 17.990 | 5 |
| AAM0370 | Sulawesi | Göteborgs naturhistoriska museum, Göteborg | Sweden | 17.991 | 9 |
| AAM0372 | Sulawesi | Museum of Comparative Zoology - Harvard University | USA | BOM1910 | 5 |
| AAM0374 | Sulawesi | Museum of Comparative Zoology - Harvard University | USA | BOM1912 | 9 |
| AAM0383 | Sulawesi | Museum of Comparative Zoology - Harvard University | USA | MCZ46401 | 5 |
| AAM0385 | Sulawesi | Naturalis Biodiversity Center, Leiden | Netherlands | RMNH.MAM.00375 | 5, 9 |
| AAM0386 | Sulawesi | Naturalis Biodiversity Center, Leiden | Netherlands | RMNH.MAM.00992 | 8 |
| AAM0396 | Buru | Naturalis Biodiversity Center, Leiden | Netherlands | RMNH.MAM.28805 | 5, 7 |
| AAM0407 | Sula or Buru Islands | Naturalis Biodiversity Center, Leiden | Netherlands | RMNH.MAM.28819 | 4 |
| AAM0408 | Sulawesi | Naturalis Biodiversity Center, Leiden | Netherlands | RMNH.MAM.43508 | 4 |
| AAM0412 | Sulawesi | Naturalis Biodiversity Center, Leiden | Netherlands | RMNH.MAM.28802 | 2 |
| AAM0413 | Sulawesi | Naturalis Biodiversity Center, Leiden | Netherlands | RMNH.MAM.00089a | 1 |
| AAM0429 | Sulawesi | Naturalis Biodiversity Center, Leiden | Netherlands | RMNH.MAM.43503[nonum06] | 5 |
| AAM0450 | Sula Islands | Natural History Museum, London | England | 19.11.23.04 | 4 |
| AAM0461 | Sulawesi | Natural History Museum, London | England | 0.3.30.19 | 2 |
| AAM0463 | Sulawesi | Natural History Museum, London | England | 9.11.30.1 | 2 |
| AAM0466 | Sula Islands | Natural History Museum, London | England | 19.11.23.08 | 3, 4 |
| AAM0473 | Sula or Buru Islands | Natural History Museum, London | England | 67.4.12.221 | 2 |
| AAM0507 | Sula Islands | Zoologische Staatssammlung München | Germany | ZSM1908_2729 | 2 |
| AAM0514 | Sula or Buru Islands | Zoologische Staatssammlung München | Germany | ZSM1949_1414 | 4 |
| AAM0526 | Sulawesi | American Museum of Natural History, New York | USA | 69415 | 1 |
| AAM0527 | Buru | American Museum of Natural History, New York | USA | 90363 | 5 |
| AAM0543 | Togean Islands | American Museum of Natural History, New York | USA | 153408 | 4 |
| AAM0561 | Sulawesi | Lee Kong Chian Natural History Museum | Singapore | ZRC.4.1953 | 2 |
| AAM0588 | Sulawesi | Naturhistoriska Riksmuseet, Stockholm | Sweden | A585922 | 2 |
| AAM0600 | Zoo | Kibun Binatang Surabaya | Indonesia | D.BB.Rusa13_10_92 | 5 |
| AAM0601 | Zoo | Kibun Binatang Surabaya | Indonesia | 19_8_98 | 1 |
| AAM0603 | Zoo | Kibun Binatang Surabaya | Indonesia | 5_6_89 | 5 |
| AAM0605 | Zoo | Kibun Binatang Surabaya | Indonesia | Pf605 | 1 |
| AAM0607 | Zoo | Kibun Binatang Surabaya | Indonesia | 19_8_88 | 1 |
| AAM0612 | Sulawesi | Naturhistorisches Museum Wien | Austria | 1493 | 4 |
| AAM0634 | Zoo | National Museum of Natural History, Washington | USA | 283108 | 1 |
| AAM0674 | Sulawesi | Zoology museum, University of Aberdeen | Scotland | RN20523 | 5 |
| AAM0734 | Sula or Buru Islands | Muséum national d’histoire naturelle, Paris | France | MNHN-ZM-AC 1880-618E | 1 |
| AAM0742 | Sula or Buru Islands | Muséum national d’histoire naturelle, Paris | France | MNHN-ZM-AC 1993-4617 | 4 |
| AAM0763 | Sulawesi | De faculteit Diergeneeskunde, Universiteit Utrecht | Netherlands | TAG_277 | 2 |
| AAM1774 | Sula or Buru Islands | Muséum des sciences naturelles, Bruxelles | Belgium | 3712 | 5 |
| AAM1794 | Sulawesi | Natural History Museum at the University of Oslo | Norway | M7237 | 5 |
| AAM0004 | Sula or Buru Islands | Zoological Museum Amsterdam | Netherlands | ZMA.MAM.00805 | 2 |
| AAM0016 | Sulawesi | Zoological Museum Amsterdam | Netherlands | ZMA.MAM.01184 | 1 |
| AAM0024 | Sulawesi | Zoological Museum Amsterdam | Netherlands | ZMA.MAM.02242 | 5 |
| AAM0025 | Sulawesi | Zool Museum Amsterdam | Netherlands | ZMA.MAM.08936 | 9 |
| AAM0031 | Sula Islands | Zoological Museum Amsterdam | Netherlands | ZMA.MAM.09116 | 2 |
| AAM0050 | Sula or Buru Islands | Zoological Museum Amsterdam | Netherlands | ZMA.MAM.25389 | 1 |
| AAM0059 | Sulawesi | Naturhistorisches Museum Basel | Switzerland | C.2882 | 2 |
| AAM0066 | Sula or Buru Islands | Naturhistorisches Museum Basel | Switzerland | C.6204 | 2 |
| AAM0068 | Sula or Buru Islands | Naturhistorisches Museum Basel | Switzerland | C.111.196 | 2 |
| AAM0080 | Sulawesi | Museum für Naturkunde, Berlin | Germany | 1969 | 9 |
| AAM0084 | Sulawesi | Museum für Naturkunde, Berlin | Germany | 8433 | 8 |
| AAM0085 | Sulawesi | Museum für Naturkunde, Berlin | Germany | 8435 | 2 |
| AAM0086 | Sulawesi | Museum für Naturkunde, Berlin | Germany | 8437_cran | 2 |
| AAM0091 | Sula or Buru Islands | Museum für Naturkunde, Berlin | Germany | 33677 | 4 |
| AAM0099 | Sulawesi | Museum für Naturkunde, Berlin | Germany | 46526 | 2, 5 |
| AAM0104 | Sula or Buru Islands | Museum für Naturkunde, Berlin | Germany | 64534 | 4 |
| AAM0109 | Sula or Buru Islands | Museum für Naturkunde, Berlin | Germany | A2620 | 1 |
| AAM0137 | Buru | Museum Zoologicum Bogoriense, Cibinong | Indonesia | 1081 | 4 |
| AAM0154 | Sulawesi | Museum Zoologicum Bogoriense, Cibinong | Indonesia | 6904 | 8 |
| AAM0158 | Buru | Museum Zoologicum Bogoriense, Cibinong | Indonesia | 6908 | 4 |
| AAM0195 | Sulawesi | Zoologisk Museum, København | Denmark | M0321 | 2 |
| AAM0198 | Sulawesi | Zoologisk Museum, København | Denmark | M1433 | 5 |
| AAM0203 | Sula or Buru Islands | Skolen for Veterinærmedicin og Husdyrvidenskab, Københavns Universitet | Denmark | Vet2 | 2, 4 |
| AAM0222 | Sulawesi | Senckenberg Naturhistorische Sammlungen Dresden | Germany | 3070 | 5 |
| AAM0231 | Sulawesi | National Museum of Scotland, Edinburgh | Scotland | NMS.Z.1878.1.4 | 5 |
| AAM0246 | Zoo | National Museum of Scotland, Edinburgh | Scotland | NMS.Z.1993.159.003 | 1 |
| AAM0252 | Zoo | National Museum of Scotland, Edinburgh | Scotland | NMS.Z.1999.185 | 2 |
| AAM0253 | Zoo | National Museum of Scotland, Edinburgh | Scotland | NMS.Z.1999.282.001 | 1 |
| AAM0267 | Zoo | National Museum of Scotland, Edinburgh | Scotland | NMS.Z.2004.161.002 | 2 |
| AAM0270 | Zoo | National Museum of Scotland, Edinburgh | Scotland | NMS.Z.2006.033.002 | 1 |
| AAM0272 | Zoo | National Museum of Scotland, Edinburgh | Scotland | NMS.Z..2006.082 | 2 |
| AAM0275 | Zoo | National Museum of Scotland, Edinburgh | Scotland | NMS.Z.2008.026. Morvell | 2 |
| AAM0283 | Sulawesi | Naturmuseum Senckenberg, Frankfurt Am Main | Germany | 423 | 2 |
| AAM0287 | Buru | Naturmuseum Senckenberg, Frankfurt Am Main | Germany | 427 | 4 |
| AAM0288 | Buru | Naturmuseum Senckenberg, Frankfurt Am Main | Germany | 429 | 5 |
| AAM0290 | Sulawesi | Naturmuseum Senckenberg, Frankfurt Am Main | Germany | 1588 | 6 |
| AAM0293 | Zoo | Naturmuseum Senckenberg, Frankfurt Am Main | Germany | 5951 | 2 |
| AAM0317 | Sulawesi | Göteborgs naturhistoriska museum, Göteborg | Sweden | 17.938 | 2 |
| AAM0319 | Sulawesi | Göteborgs naturhistoriska museum, Göteborg | Sweden | 17.940 | 2 |
| AAM0323 | Sulawesi | Göteborgs naturhistoriska museum, Göteborg | Sweden | 17.944 | 5 |
| AAM0326 | Sulawesi | Göteborgs naturhistoriska museum, Göteborg | Sweden | 17.947 | 2, 9 |
| AAM0333 | Sulawesi | Göteborgs naturhistoriska museum, Göteborg | Sweden | 17.954 | 5 |
| AAM0334 | Sulawesi | Göteborgs naturhistoriska museum, Göteborg | Sweden | 17.955 | 5 |
| AAM0336 | Sulawesi | Göteborgs naturhistoriska museum, Göteborg | Sweden | 17.957 | 1 |
| AAM0337 | Sulawesi | Göteborgs naturhistoriska museum, Göteborg | Sweden | 17.958 | 6 |
| AAM0339 | Sulawesi | Göteborgs naturhistoriska museum, Göteborg | Sweden | 17.960 | 9 |
| AAM0340 | Sulawesi | Göteborgs naturhistoriska museum, Göteborg | Sweden | 17.961 | 2 |
| AAM0347 | Sulawesi | Göteborgs naturhistoriska museum, Göteborg | Sweden | 17.968 | 2 |
| AAM0357 | Sulawesi | Göteborgs naturhistoriska museum, Göteborg | Sweden | 17.978 | 1, 9 |
| AAM0365 | Sulawesi | Göteborgs naturhistoriska museum, Göteborg | Sweden | 17.986 | 5 |
| AAM0369 | Sulawesi | Göteborgs naturhistoriska museum, Göteborg | Sweden | 17.990 | 5 |
| AAM0370 | Sulawesi | Göteborgs naturhistoriska museum, Göteborg | Sweden | 17.991 | 9 |
| AAM0372 | Sulawesi | Museum of Comparative Zoology - Harvard University | USA | BOM1910 | 5 |
| AAM0374 | Sulawesi | Museum of Comparative Zoology - Harvard University | USA | BOM1912 | 9 |
| AAM0383 | Sulawesi | Museum of Comparative Zoology - Harvard University | USA | MCZ46401 | 5 |
| AAM0385 | Sulawesi | Naturalis Biodiversity Center, Leiden | Netherlands | RMNH.MAM.00375 | 5, 9 |
| AAM0386 | Sulawesi | Naturalis Biodiversity Center, Leiden | Netherlands | RMNH.MAM.00992 | 8 |
| AAM0396 | Buru | Naturalis Biodiversity Center, Leiden | Netherlands | RMNH.MAM.28805 | 5, 7 |
| AAM0407 | Sula or Buru Islands | Naturalis Biodiversity Center, Leiden | Netherlands | RMNH.MAM.28819 | 4 |
| AAM0408 | Sulawesi | Naturalis Biodiversity Center, Leiden | Netherlands | RMNH.MAM.43508 | 4 |
| AAM0412 | Sulawesi | Naturalis Biodiversity Center, Leiden | Netherlands | RMNH.MAM.28802 | 2 |
| AAM0413 | Sulawesi | Naturalis Biodiversity Center, Leiden | Netherlands | RMNH.MAM.00089a | 1 |
| AAM0429 | Sulawesi | Naturalis Biodiversity Center, Leiden | Netherlands | RMNH.MAM.43503[nonum06] | 5 |
| AAM0450 | Sula Islands | Natural History Museum, London | England | 19.11.23.04 | 4 |
| AAM0461 | Sulawesi | Natural History Museum, London | England | 0.3.30.19 | 2 |
| AAM0463 | Sulawesi | Natural History Museum, London | England | 9.11.30.1 | 2 |
| AAM0466 | Sula Islands | Natural History Museum, London | England | 19.11.23.08 | 3, 4 |
| AAM0473 | Sula or Buru Islands | Natural History Museum, London | England | 67.4.12.221 | 2 |
| AAM0507 | Sula Islands | Zoologische Staatssammlung München | Germany | ZSM1908_2729 | 2 |
| AAM0514 | Sula or Buru Islands | Zoologische Staatssammlung München | Germany | ZSM1949_1414 | 4 |
| AAM0526 | Sulawesi | American Museum of Natural History, New York | USA | 69415 | 1 |
| AAM0527 | Buru | American Museum of Natural History, New York | USA | 90363 | 5 |
| AAM0543 | Togean Islands | American Museum of Natural History, New York | USA | 153408 | 4 |
| AAM0561 | Sulawesi | Lee Kong Chian Natural History Museum | Singapore | ZRC.4.1953 | 2 |
| AAM0588 | Sulawesi | Naturhistoriska Riksmuseet, Stockholm | Sweden | A585922 | 2 |
| AAM0600 | Zoo | Kibun Binatang Surabaya | Indonesia | D.BB.Rusa13_10_92 | 5 |
| AAM0601 | Zoo | Kibun Binatang Surabaya | Indonesia | 19_8_98 | 1 |
| AAM0603 | Zoo | Kibun Binatang Surabaya | Indonesia | 5_6_89 | 5 |
| AAM0605 | Zoo | Kibun Binatang Surabaya | Indonesia | Pf605 | 1 |
| AAM0607 | Zoo | Kibun Binatang Surabaya | Indonesia | 19_8_88 | 1 |
| AAM0612 | Sulawesi | Naturhistorisches Museum Wien | Austria | 1493 | 4 |
| AAM0634 | Zoo | National Museum of Natural History, Washington | USA | 283108 | 1 |
| AAM0674 | Sulawesi | Zoology museum, University of Aberdeen | Scotland | RN20523 | 5 |
| AAM0734 | Sula or Buru Islands | Muséum national d’histoire naturelle, Paris | France | MNHN-ZM-AC 1880-618E | 1 |
| AAM0742 | Sula or Buru Islands | Muséum national d’histoire naturelle, Paris | France | MNHN-ZM-AC 1993-4617 | 4 |
| AAM0763 | Sulawesi | De faculteit Diergeneeskunde, Universiteit Utrecht | Netherlands | TAG_277 | 2 |
| AAM1774 | Sula or Buru Islands | Muséum des sciences naturelles, Bruxelles | Belgium | 3712 | 5 |
| AAM1794 | Sulawesi | Natural History Museum at the University of Oslo | Norway | M7237 | 5 |
3 Results
The babirusa skulls exhibited a range of anomalies of maxillary canine tooth growth that could be grouped under eight headings. Some skulls featured under more than one heading.
3.1 Failure of the tooth alveolus to rotate normally
Fifteen skulls showed various amounts of reduced tooth rotation reflecting anomalous alveolar rotation in a median plane (Fig. 1). In thirteen skulls (three from Buru and the Sula Islands and ten from Sulawesi) both maxillary canine teeth were orientated more rostrally than 70 degrees with respect to the hard palate; the mean angle of rotation was 45 ± 12.4 degrees and ranged from 10 to 60 degrees. Two Sulawesi skulls each had one tooth orientated at 70 and 75 degrees respectively. The Sulawesi skull illustrated in Fig. 1A showed the lowest value for tooth rotation found. Although the external structures of the alveoli in this skull were orientated at approximately 45 degrees to the hard palate, the angles of curvature of the teeth within the alveoli were orientated rostro-ventrally. In another skull (AAM0050, from Buru or the Sula Islands), the right canine grew with a caudally orientated curvature, even though the alveolus was orientated at 10 degrees rostrally with respect to the hard palate. The flattened bony bracket along the caudal edge of the alveolus of these skulls appeared somewhat reduced in size (Fig. 1B).
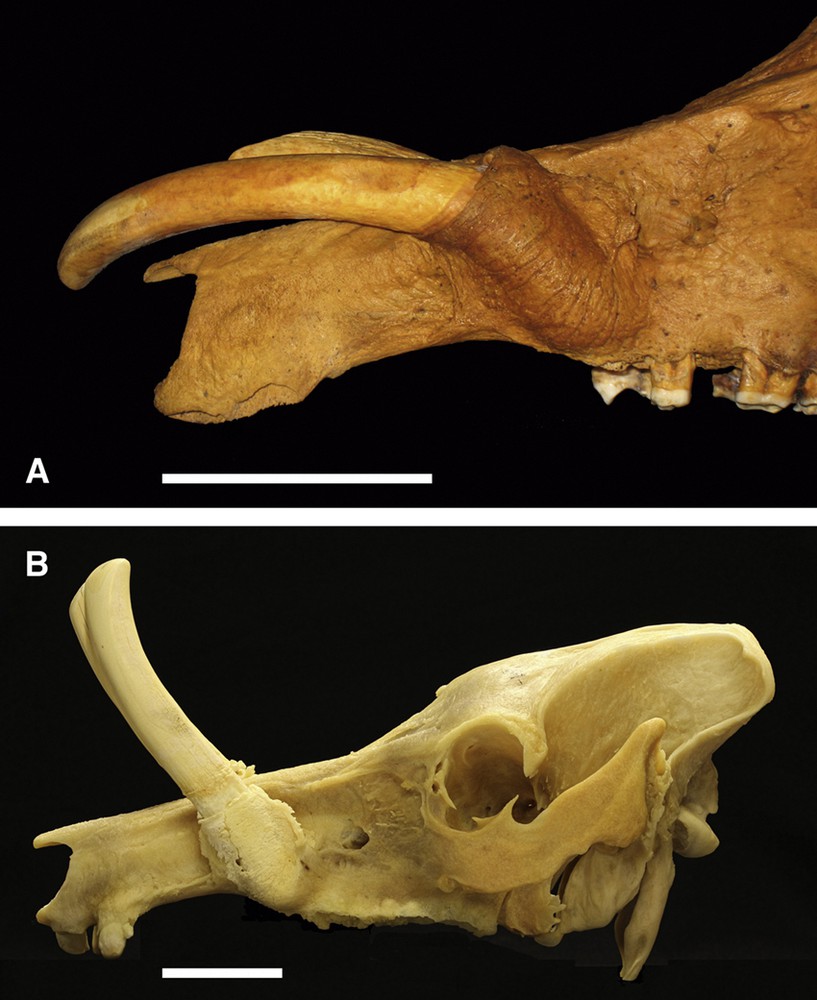
A. Left lateral view of a babirusa skull (AAM0605) from an Indonesian zoo (and originating from Sulawesi) illustrating failure of the maxillary canine tooth alveolus to rotate normally and showing the orientation of the growth of these teeth to aberrantly point rostro-ventrally. Note that the tips of the teeth have been cut off for husbandry reasons. B. Left lateral view of a babirusa skull (AAM0253) from a European zoo (originating from Sulawesi) illustrating incomplete rotation of the maxillary canine tooth alveolus. Note that the tips of the teeth have been cut off for husbandry reasons (scale = 50 mm).
3.2 Anomalous alignment of the tooth with the median plane of the cranium
There were 29 skulls in which one or both maxillary canine teeth did not grow (more or less) symmetrically towards the median plane of the cranium (Fig. 2). In most cases [16] the left canine tooth grew to lie over the right side of the skull (Fig. 2A); five of these skulls were from Buru or the Sula Islands and 11 from Sulawesi. In eight cases the teeth crossed one another; one from the Sula Islands and seven came from Sulawesi. In four cases the right canine tooth grew over to the left side of the face; one from Buru or the Sula Islands and three skulls were from Sulawesi. On one occasion (Sulawesi skull) the pattern of growth taken by the right maxillary canine tooth was more complex (Fig. 2B).
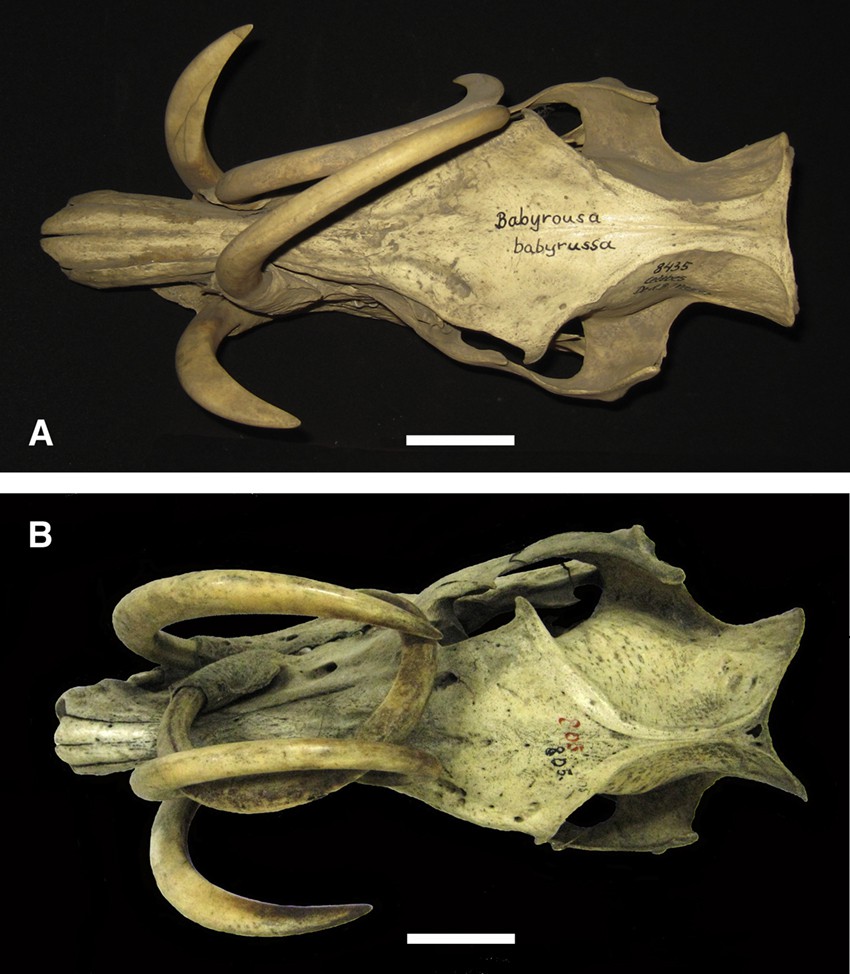
A. Dorsal view of a Sulawesi babirusa skull (AAM0085) illustrating growth of both maxillary canine teeth to the right side of the head rather than to the median plane. B. Dorsal view of the skull (AAM0004) of a babirusa from Buru or the Sula Islands showing a normally grown left maxillary canine tooth and an aberrantly grown right maxillary canine tooth (scale = 50 mm).
3.3 Failure of the tooth to erupt through the skin
In one skull from the Sula Islands the tight, curve-linear shape and the white superficial appearance of the left maxillary canine tooth indicated that it had not passed rostrally through the skin during its growth (Fig. 3). The curved shaft of the tooth projected rostrally, and was ridged along its caudal border. The tip of the tooth had penetrated the left nasal bone and grown into the nasal cavity. The open apex of the tooth had grown caudally into the maxilla. There was no alveolar bone ventral, rostral, lateral and medial to the tooth. Some alveolar bone, largely comprising the flattened caudally facing bracket, was present around the tooth's open apex and adjacent to the caudal edge of the tooth. The right canine tooth had grown normally to a length of 85 mm above the skin (Fig. 3).

Left lateral view of a babirusa skull (AAM0466) from the Sula Islands illustrating a maxillary canine tooth tightly curled and inserting its tip through the left nasal bone into the nasal cavity. Note its bony alveolar support is restricted to the caudal surface, which shows corrugations (scale = 50 mm).
3.4 Canine tooth erosion of the nasal bones
Fourteen skulls showed evidence that the tips of one or both maxillary canine teeth had eroded the nasal bones (Fig. 4). Eleven of these skulls were from Buru or the Sula Islands, and three were from Sulawesi. On six occasions, five being skulls from Buru, the tip of the tooth had penetrated the nasal bone and entered the nasal cavity (Fig. 4). The open apex of the tooth that had punctured the nasal bone appeared to have also extended the supporting alveolus caudally and in three cases eroded the integrity of the caudo-ventral surfaces of the alveolus (Fig. 4A). Those canine teeth that had not penetrated the nasal bones appeared to have normal alveoli.

A. Left lateral view of the skull (AAM0091) of a babirusa from Buru or the Sula Islands in which the left maxillary canine tooth has eroded and penetrated the left nasal bone. Note the disruption of the rostral aspect of the alveolus. Note also the ventro-caudal growth and exposure of the apex of the tooth. B. Dorsal view of the cranium (AAM0137) of a relatively young adult male babirusa from Buru demonstrating the erosion and penetration of the bones at the naso-frontal suture (scale = 50 mm).
3.5 Canine tooth erosion of the frontal bones
There were 22 skulls in which the maxillary canine teeth had eroded the frontal bones; four were from Buru and the Sula Islands and 18 were from Sulawesi and the Togian Islands. On 12 occasions the tip of the tooth had penetrated the bone and entered the frontal sinuses (Fig. 5). Three of these skulls were from Buru or the Sula Islands, and nine were from Sulawesi or the Togian Islands. On one occasion the damage was caused by the tooth on the right side of the skull reaching over the face to insert into the left frontal bone. In all cases the alveoli appeared to be normal.
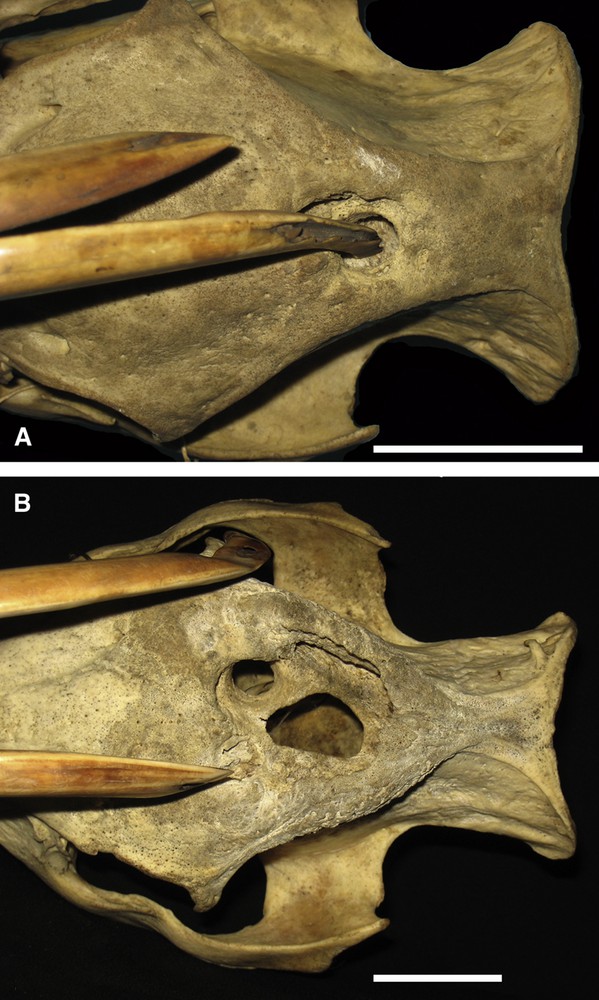
A. Dorsal view of the cranium (AAM0231) of a Sulawesi babirusa illustrating the erosion of the frontal-parietal suture area and the penetration by the left maxillary canine tooth into the frontal sinuses. Note the area of superficial bone erosion around the tooth, and the size and shape of the puncture relative to the size of the tooth. B. Dorsal view of the cranium (AAM0674) of a Sulawesi babirusa illustrating the extensive erosion of the left and right frontal bones and two of the three penetrations into the frontal sinuses. There was no penetration of the cranial cavity (scale = 50 mm).
3.6 Canine tooth erosion of the parietal bones
There were two skulls from Sulawesi where one of their maxillary canine teeth had eroded a parietal bone.
3.7 Supernumerary maxillary canine tooth
One skull from Buru Island had two maxillary canines arising from an adjacent pair of alveoli on the left side of the cranium (Fig. 6). The more rostral tooth was larger than caudo-lateral tooth (Fig. 6A), and the oval diameters of its alveolus were correspondingly larger. The external lengths of the two adjacent alveoli were similar (Fig. 6B). One maxillary canine tooth arose from the right alveolus (Fig. 6A). It was of approximately the same size as the more rostral tooth on the left side of the cranium. The tips of all three maxillary canine teeth had broken, and one had eroded and penetrated the right frontal bone (Fig. 6A).

A. Dorsal view of the cranium (AAM0396) of a Buru Island babirusa with two maxillary canine teeth on the left and one maxillary canine tooth on the right side of the cranium. Each canine tooth has an alveolus. Note the erosion of the right frontal bone. B. Left lateral view of the alveoli (AAM0396) on the left of the cranium illustrating their approximately similar lengths and the single caudal flattened, bony bracket.
3.8 Absence of maxillary canine tooth
Three Sulawesi skulls exhibited alveoli (two right, one left) with no formed maxillary canine teeth in them. Each alveolus was short in vertical length, appeared to have rotated normally, had an apparently normal, caudally orientated flattened bony bracket on its caudal surface, and a smaller cranial bracket. Each alveolus was somewhat flattened latero-medially. In each case the rostrally orientated remains of the alveolar lumen was filled with spongy bony.
No babirusa skull exhibited a maxillary canine tooth penetrating the eye socket.
4 Discussion
This is the first detailed analysis of aberrations in the growth of maxillary canine teeth growth in the male babirusa. It draws on the evidence contained in skeletal tissues gathered, stored and curated in museum collections around the world. These skulls serve as a conserved sample of the range of tooth problems to be found naturally in these animals.
A number of skulls variously showed the effect on maxillary canine growth of a failure by the alveolus to rotate into its normal position [9]. These represented a relatively small number of the aberrant skulls identified (18.1%) and only 3.5% of the 431 skulls surveyed. The underlying cause of this anomaly remains uncertain. Physical resistance to rotation may be hypothesized, but there was no structural evidence present that could be interpreted as giving support to this notion. It had been suggested that normally the alveolus was slowly “pulled” into position by fascia associated with the growth of the skull during juvenile development [9]; the sutures of the skull are major sites of bone expansion during postnatal craniofacial growth [18,19]. In almost all, fourteen, of the cases the relatively small size of the caudal bracket on the alveolus might indicate supportive evidence. However, in one of the other skulls (Fig. 1A) the relatively large caudal alveolar bracket would appear not to be consistent with this possibility. The partial rotation of the alveolus initially suggested the possibility of traumatic interference with this hypothesized mechanism. However, the bilateral failure of rotation seen in ten of the skulls would appear to argue against this possibility.
One of the zoo specimens in the present study (AAM0605) together with another one published by Grzimek [11] and Mohr [12] illustrated an additional component of this anomaly. The alveolus pointed partially rostrally, but the canine tooth grew rostro-ventrally (Fig. 1A). This suggested that one component playing a role in the growth of the tooth might be the post-alveolar weight of the canine tooth itself. Perhaps in the relatively extreme situation of rostrally orientated teeth there is enough gravitational leverage of the erupted tooth to have an effect on the odontoblasts in the apical region of the alveolus; clearly more dentine tissue was laid down linearly on the dorsal side of the tooth than on the ventral side and so the tooth grew ventrally (Fig. 1A). Recent studies have shown that microgravity and applied pressures contribute to the bioengineering of tooth tissue [20,21].
Abnormal alignment of the maxillary canine teeth with respect to the median plane was recognized as such in 29 skulls. These represented 34.9% of the anomalous sample, and 6.7% of all the skulls observed. Various suggestions would seem to indicate explanations for the range of variation seen. Failure of the maxillary canine teeth to meet appropriately in the median plane may well have resulted in teeth crossing over one another. A small wear pattern has been reported on the adjacent medial surfaces of normally grown maxillary canine teeth [8]. Trauma on one side may have led to partial disorientation of one tooth (Fig. 2A). It was earlier suggested that because Babirusa rub their canine teeth against the walls of their pens, and even the legs of their keepers they thereby modify the direction of growth of the teeth [22,23].
Initially it was puzzling to explain the circumstances that brought about the sinuous path taken by the right maxillary canine tooth in Fig. 2B. However, one possible suggestion was that the right canine tooth grew under the growth path of the left canine tooth and that pressure exerted on the former's lateral surface proximally and distally induced the growth pattern shown (Fig. 2B). Its alveolus appeared normal externally.
Thirty-six of the skulls showed tooth-induced erosion of the bones of the cranium, and this anomaly represented 8.3% of the 431 adult male babirusa skulls examined. The skulls that had maxillary canine teeth penetrating the nasal bones were mainly from Buru and the Sula Islands (11 of 14), whereas the skulls with teeth penetrating the frontal and parietal bones were mainly from Sulawesi and the Togian Islands (18 of 22). These differences reflected the geographically based difference in patterns of normal growth of the maxillary canine teeth [10]. An additional seventeen skulls (3.9%), all from Sulawesi, had maxillary canine teeth that showed anatomical signs of potential future damage to the cranial bones, interrupted by having been trapped and killed by local people. Comparable images, of maxillary canine teeth tips resting on the forehead skin of babirusa, were seen in video recordings made in North Sulawesi [8,24] (Macdonald, unpublished). Thus, when taken together these data suggested that approximately 12% of the adult male babirusa in the wild would experience erosion of the cranial bony tissues as a result of maxillary canine tooth growth.
This incidence of damage to the nasal, frontal and parietal bones of the adult male babirusa skull by the growing maxillary canine teeth was greater than expected. The published literature had only made mention of two examples [13,25,26]. One of these, the rotation of the maxillary tooth illustrated in Fig. 3, was likely to have been caused by the failure of the tooth to penetrate the skin. It is unclear if this was due to insufficient hardness of the tip. The tip of the juvenile Sus scrofa maxillary canine tooth is initially covered in enamel [27], but it is not yet known if this is the case in babirusa [10]. An alternative suggestion was that “over rotation” of the alveolus may have occurred such that the tip of the tooth was carried past the appropriate angle to pierce the skin. However, the appearance of the remaining elements of the alveolus would seem to argue against this possibility (Fig. 3). Another hypothesis was that there had been greater resistance of the skin to being punctured. Nevertheless, the contralateral tooth grew normally. The observation that the tip of the left canine tooth penetrated and was then mechanically caught in the left nasal bone, and yet continued to grow in a curve, supported the hypothesis that there was some form of pressure/resistance feedback mechanism to the tooth growth cell multiplication region within the alveolus [10]. The absence of skin or bone pressure on the tip of the contralateral tooth seemed to have enabled a more linear pattern of growth as the normal result.
The caudal direction of growth shown by the open apex of the left maxillary canine tooth (Fig. 3) was comparable to that shown by the aberrant growth of a babirusa mandibular canine tooth reported by Meyer [25]; he drew attention to a right canine tooth that had grown in a complete circle and re-entered the jaw (Fig. 7A). It appeared to have had a tight curve-linear growth pattern from early in its development. This pattern had continued through adulthood with the tip of the tooth remaining inside the oral cavity, where it first grew dorsally, then caudally before pointing ventrally, lateral to itself. Within the jaw the tooth tip had overlapped and insinuated itself into its own tooth shaft. The growth of the tooth, at its open apex, appeared to have forced that end of the tooth to move caudally through the bony alveolar tissue of the mandible and had caused it to partially erupt from the lateral surface of the mandible (Fig. 7B).
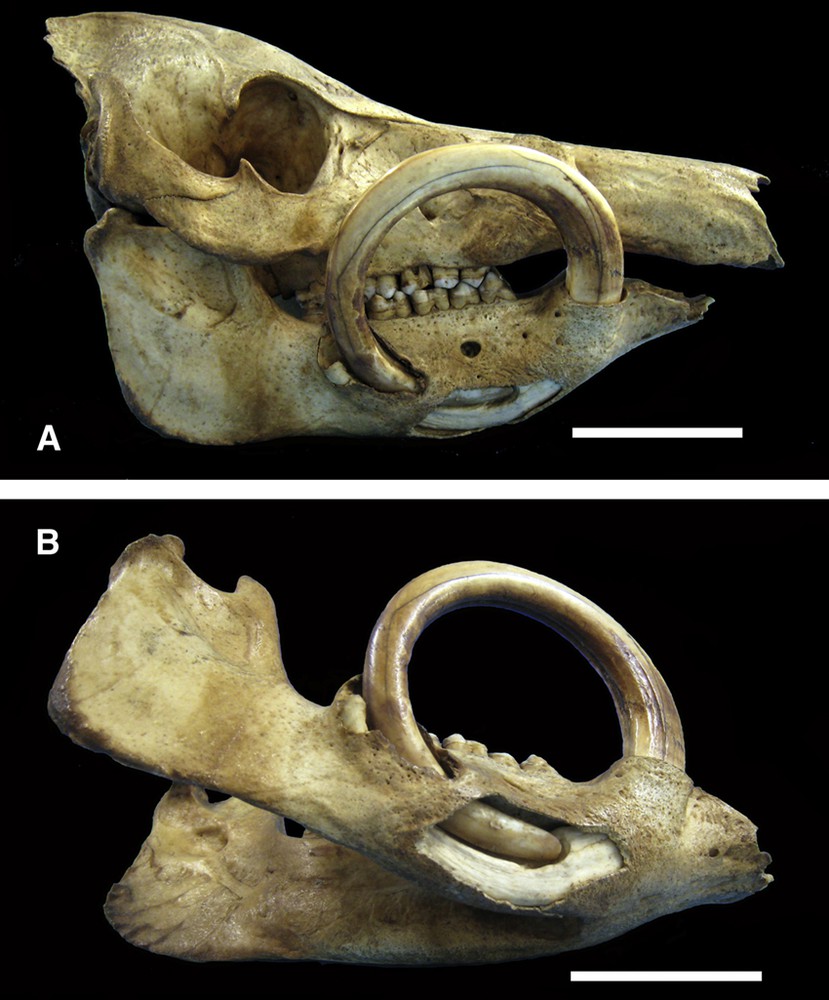
A. Right lateral view of the skull (AAM0385) of a Sulawesi babirusa illustrating the curved path taken by the growing mandibular canine tooth. Note the penetration by the tooth apex of the bony mandibular tissue caudal to the encircling shaft of the tooth. B. Right ventro-lateral view of the mandible (AAM0385) of the same Sulawesi babirusa with a window cut into the bony mandibular tissue to reveal the path taken by the tip of the tooth through the bone and into the shaft of the tooth itself. The apex of the tooth has grown through the lateral bony tissue of the mandible and lies caudal to the curved shaft of the tooth (scale = 50 mm).
Ten additional examples of caudal growth of the open apex of the mandibular canine tooth through the mandibular periosteum were noted in the present study (Table 1); in each case that part of the tooth had eroded through the supporting bony tissue of the lateral surface of the mandible and partially or completely entered the overlying connective tissue space. These findings would seem to support the suggestion that the physical resistance to growth of the tooth in the normal rostral-dorsal direction had been greater than that which normally retained the open apex of the tooth within the cortex of the mandible, and had thus led to its extension caudo-dorsally. Caudal growth of the canine tooth apex has been recorded in the narwhal during normal growth [28].
Bilateral anomalous circular growth of the mandibular canine teeth of Sus scrofa, back into and through the mandible, has been described by Cheselden [29] and Miles [26]. Similarly overgrown canine teeth have been deliberately produced by human removal of the upper canines in young domesticated male pigs [30]. Anomalous growth of a mandibular canine tooth has also been recorded in an Arizona peccary (Pecari tajacu) in which the left canine tooth was described as having split into three parts [31]. Re-analysis by Miles and Grigson [32] led them to conclude that there had been two tooth germs, the apexes of which had fused. The illustration suggested that the tooth root had rotated on its axis and its “combined apex” had become seated ventrally in the mandible rather than caudally [31]. The two unequal parts of this combined tooth curved rostrally and appear to have pierced ventrally out of the mandible; one additional malformed part did rise dorsally. Recent reviews of the human and animal literature suggest that many of the mechanisms controlling normal tooth growth remain to be elucidated [33–35].
It has been recognized for some time that the skulls of babirusa from Buru and the Sula islands are smaller than those from Sulawesi [2,12]. It was also recognized that the canine teeth of babirusa from Buru and the Sula islands tended to be slenderer than those from Sulawesi, which are larger and longer in size [2]. This enlarged size together with their pattern of angular growth [10] seemed to explain why the maxillary canine teeth of Sulawesi skulls generally reached over the nasal bones and eroded and penetrated some of the skulls more caudally than those from Buru and the Sula Islands.
A number of the skulls showed very well-defined penetrations of the skull that closely matched the sizes of the penetrating teeth (Figs. 3 and 4). Other skulls had large areas of bony erosion around penetration sites that were much larger than the diameter of the causal tooth (Figs. 5A and B). The latter suggested that the animal had experienced a relatively long period of pressure on the cranial tissues from its canine tooth. The size of the hole also indicated that the animal had rubbed its head and the tooth for a long period of time causing the latter to create extensive destruction of specific areas of the cranium. Normally the adult male rubbed the side of his face against small diameter trees and saplings to deposit olfactory secretions from the glands in his eye sockets and from its mouth [5,6]. He also ploughed his nose and face into soft and wet substrate [8,36] as well as rubbed the side of his face against stones and the sides of wallows [37]. These caused wear of the maxillary canine teeth particularly on their lateral side towards the tips of the teeth [8]. Often this wear was sufficient to cause the teeth to thin and break, resulting in a sharp point or flat edge (Fig. 2A, Fig. 4, Fig. 6A). The erosion of the frontal and parietal bones would appear to have occurred following such breakage. The relatively large amount of mobility of the maxillary canine tooth within its alveolus was likely to be the cause of the large area of bony erosion. The maxillary alveolus provided about 50 mm of support, but the exposed tooth could be over 400 mm long; 432 mm and 425 mm are the two longest measurements of maxillary canine teeth recorded from Sulawesi babirusa [38]. By way of contrast, the mandibular alveolus provided substantially better support, by quite tightly holding about 50% of the length of the mandibular canine tooth.
Supernumerary maxillary canine teeth would appear to be rare in babirusa (Fig. 6). We did not investigate whether other, non-canine supernumerary teeth were present in the skulls under investigation. In a smaller survey of babirusa, none were reported by Miles and Grigson [32], although they did comment upon and depict a domestic pig (Sus scrofa) with two mandibular canine teeth on each side. They also indicated that there have been a number of descriptions of bilateral paired tusks (incisors) in African elephants (Loxodonta africana). Two walrus (Odobaenus rosmarus) have been found with supernumerary maxillary canine teeth on one side of the skull [39], and an instance of duplication of both maxillary canine teeth has also been reported [40].
Clearly the age of the babirusa was likely to be an important factor in the incidence of those tooth aberrations where erosion of the frontal and parietal bones had occurred. As the adult male babirusa grows older the length of the maxillary canine teeth grow longer. Methods are available for determining the ages of wild bushpig (Potamochoerus larvatus) [41], Eurasian wild pig (Sus scrofa) [42], and warthog (Phacochoerus africanus) [43]. However, there are as yet no clearly defined ways of aging babirusa from the wild. The presence of babirusa of Sulawesi origin in international zoological collections offers the opportunity to monitor and describe age changes in canine tooth growth [17]. It is recognized that there will be some form of impact of zoo diet, veterinary care and general animal management on tooth growth (Fig. 1B). It is also well known that most animal species in zoological collections tend to live longer than those in the wild [44]. Currently, male babirusa in zoos are living over 12 years (when fecundity starts to markedly decline) up to 19 years of age [17]. Veterinary awareness of the possibility that the maxillary canine might grow into the forehead of the animal, and mindfulness of the brittle nature of the babirusa's canine teeth [12], has for many years led to precautionary trimming of those teeth in zoos (Fig. 1B). Modern veterinary anaesthetic procedures facilitate such procedures and as a result, the maxillary canine teeth are cut as a precaution in many zoological collections [14,15]. Failure of canine teeth to erupt through the skin (Fig. 3) has occurred in zoological collections, and has been surgically treated successfully. Treatment of injured canine teeth has also been effective [15,44]. Although somewhat flattened and filled with spongy bone, the three maxillary canine alveoli that were found empty of teeth had dorsal dimensions and structure that suggested each one had formerly accommodated a canine tooth. Concern about the possibility of infection of the pulp cavity of the maxillary canine teeth has been alleviated by awareness that normal marking behaviour often results in sufficient tooth wear to expose the pulp cavity (Figs. 5 and 6) [8].
5 Conclusions
Most adult male babirusa have normally orientated maxillary canine teeth. Approximately 12% of the animals in the wild experience erosion of the cranial bony tissues as a result of maxillary canine tooth growth. An additional group of babirusa (ca. 4%) escaped this problem due to the relative fragility of the dentine of their teeth and the tooth wear caused by their head-rubbing, scent-marking behaviour.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgements
The author gratefully acknowledges the kind hospitality and support of Friederike Johansson, Göran Nilson and Bianca Ziehmer, and the assistance with French translation provided by Baptiste Mulot. He would also like to thank the curators and staff of the following museums for access to the babirusa skeletal material that form part of their collections:
Zoologisk Museum, København, Denmark; University Museum of Zoology, Cambridge, England; Natural History Museum, London, England; Oxford University Museum of Natural History, Oxford, England; Museum für Naturkunde, Berlin, Germany; Senckenberg Naturhistorische Sammlungen Dresden, Germany; Naturmuseum Senckenberg, Frankfurt Am Main, Germany; Zoologische Staatssammlung München, Germany; Private H.M. Collection, Bogor, Indonesia; Museum Zoologicum Bogoriense, Cibinong, Indonesia; Museum Wallacea, Universitas Haluoleo, Kendari, Indonesia; Universitas Tadulako, Palu, Indonesia; Zoological Museum Amsterdam, The Netherlands; Naturalis Biodiversity Center, Leiden, The Netherlands; National Museum of Scotland, Edinburgh, Scotland; Göteborgs naturhistoriska museum, Sweden; Naturhistoriska Riksmuseet, Stockholm, Sweden; Naturhistorisches Museum Basel, Switzerland; The Field Museum, Chicago, USA; American Museum of Natural History, New York, USA; National Museum of Natural History, Washington, USA.
He is grateful to the University of Edinburgh and the Balloch Trust for financial support during these studies.


