1 Introduction
The babirusa (Babyrousa spp.), a suid, is found in Eastern Indonesia, on the island of Sulawesi [1], the Togian Islands [2], Buru [3] and the Sula Islands of Sehu, Taliabu and Mangole [4]. Recent anatomical investigations have focused on the growth of the canine teeth in the babirusa [5], and on the causes of wear of those teeth [3,6]. However, the general aspects of the anatomy and wear of babirusa cheek teeth have not yet been described. Questions that have been raised include whether this wear is similar to, or different from that experienced by wild and feral Sus scrofa [7–10]; might differences in diet play a role in the abrasion experienced by the cheek teeth of these two genera of pigs? It has also been reported that the babirusa on the northern peninsula of Sulawesi regularly ingest volcanic mud [11,12]. Might this behaviour have an impact on the wear pattern of the cheek teeth of these babirusa? There have been no reports from Buru of comparable consumption of inorganic material by the babirusa there [3], so are the cheek-teeth wear patterns on that island in some way different?
Patterns of wear of the cheek teeth have been used for some time to construct methods for estimating the age of wild Sus scrofa [7,13]. Various ways have been devised for extending these methods to determine the chronological ages of recent and fossilised domestic Sus pigs [9,10]. Tooth age determination studies have also been carried out on the Warthog Phacochoerus africanus [14], Bushpig Potamochoerus porcus [15] and the Forest Hog Hylochoerus meinezhargeni [16]. Recent studies of the comparative lengths of the continually growing maxillary canine teeth of the male babirusa have provided an indication of the relative ages of B. babyrussa, from Buru and the Sula Islands, and B. celebensis from Sulawesi (Supplementary Figs. 1 & 2); the skulls were divided into five ‘age categories’ (A–E) [17]. Based on these criteria, the corresponding wear of the cheek teeth in the two species was investigated. The term ‘pillar’ (Fig. 1) was applied to each tooth and used to define their division into the one, two or three cusp-regions that exhibited wear [10].

Cheek tooth wear patterns of babirusa at different ages: (a) the right mandible (AAM0294) from Buru illustrating maxillary canine tooth age (A); (b) the right mandible (AAM0355) from Sulawesi illustrating maxillary canine tooth age (C); (c) the right mandible (AAM0365) from Sulawesi illustrating maxillary canine tooth age (E) (scale = 10 mm).
2 Materials and methods
The wear on the occlusal surfaces of male babirusa cheek teeth was evaluated in 53 skulls of Babyrousa babyrussa and 87 skulls of B. celebensis (Supplementary Tables 1 and 2). Some crania did not have mandibles, and vice versa. The method used was a modification of that originally proposed for Sus scrofa [7] and subsequently modified [10]. Codes were defined for each pillar of the mandibular and maxillary permanent third premolar (P3) and fourth premolar (P4), first permanent molar (M1), second permanent molar (M2) and third permanent molar (M3) teeth. The P3 and P4 have one pillar (comprising one cusp each), the M1 and M2 have two pillars each (comprising two cusps each), and the M3 has three pillars (comprising two cusps and a caudal single cusp) (Fig. 1). The digit ‘1′ referred to no dentine exposure - enamel wear only; the digit ‘2′ indicated that dentine was exposed as one or more small unconnected area(s) on the occlusal surface; the digit ‘3′ signified that dentine was exposed as a single area occupying most of the occlusal surface; and the digit ‘4′ signalled that enamel on part or all of the pillar edge has worn away (Fig. 2). Codes were also used to describe the connection between pillars on each tooth. The symbol ‘/’ signified that the tooth pillars were separated by an enamel bridge; and the symbol ‘–’ signified that tooth pillars were joined by dentine exposure.
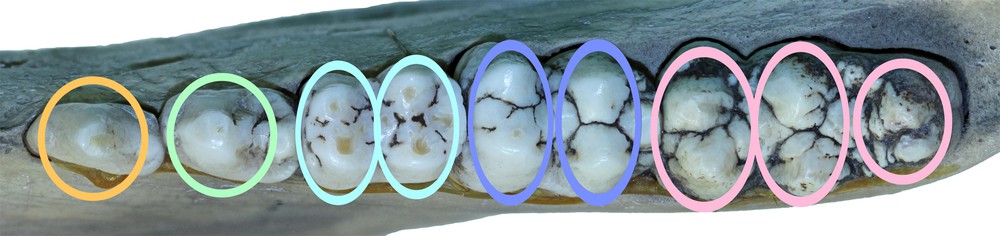
The cheek teeth of a right mandible from Buru (AAM0294) with coloured ovals superimposed to indicate the structures referred to by the term ‘pillar′ in this paper.
3 Results
There was no significant difference between the tooth wear patters of skulls in groups A and B; these were amalgamated to form the group A + B. Similarly, for groups C and D, the results for these skulls were merged to form group C + D. This procedure was carried out for the results of both species of babirusa.
The tooth wear patterns observed were tabulated and summarised in Figs. 3–6. There was close correspondence in wear patterns between each side of the mouth in both species and in all age groups. The wear patterns of the mandibular and maxillary teeth, although not identical, were very similar.
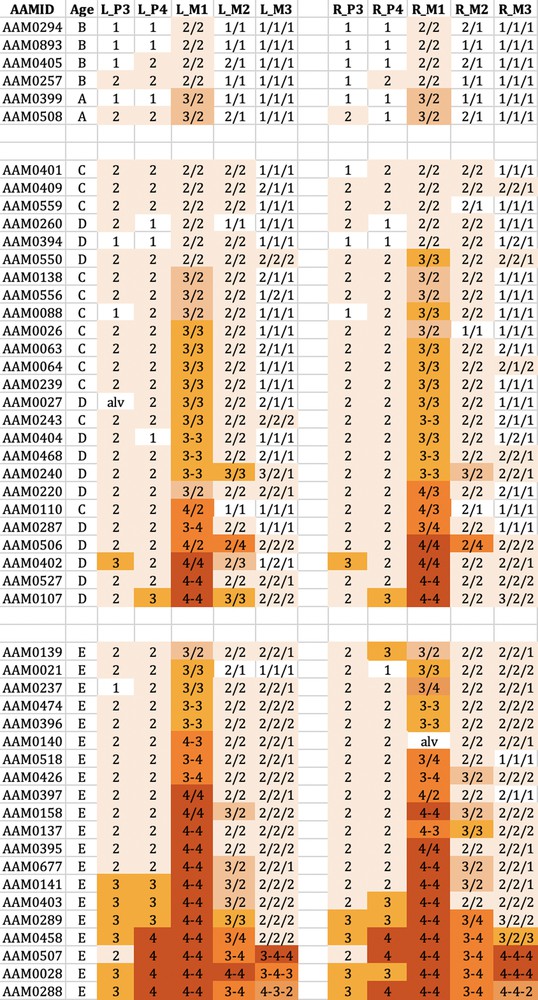
The wear observed in the mandibular cheek teeth of babirusa skulls from Buru. Age refers to the maxillary canine tooth age (A–E). L_P3 = left premolar 3; L_P4 = left premolar 4; L_M1 = left molar 1; L_M2 = left molar 2; L_M3 = left molar 3. R_P3= right premolar 3; R_P4 = right premolar 4; R_M1 = right molar 1; R_M2 = right molar 2; R_M3 = right molar 3. The digit ‘1′ referred to no dentine exposure - enamel wear only; ‘2′ indicated that dentine was exposed as one or more small unconnected area(s) on the occlusal surface; ‘3′ signified that dentine was exposed as a single area occupying most of the occlusal surface; ‘4′ signalled that enamel on part or all of the pillar edge has worn away. The symbol ‘/’ signified that the tooth pillars were separated by an enamel bridge; and the symbol ‘–’ signified that tooth pillars were joined by dentine exposure.
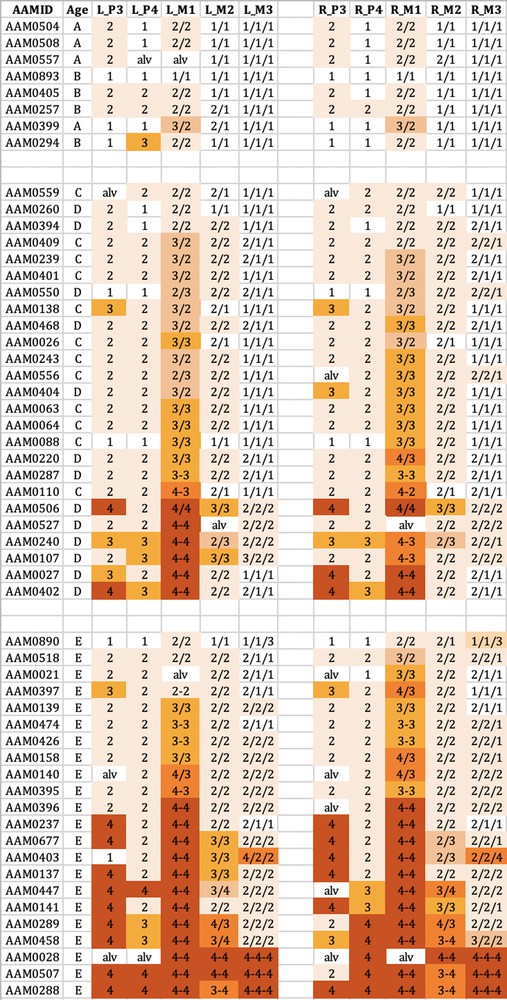
The wear observed in the maxillary teeth of babirusa skulls from Buru. The symbol nomenclature is as in Fig. 3.
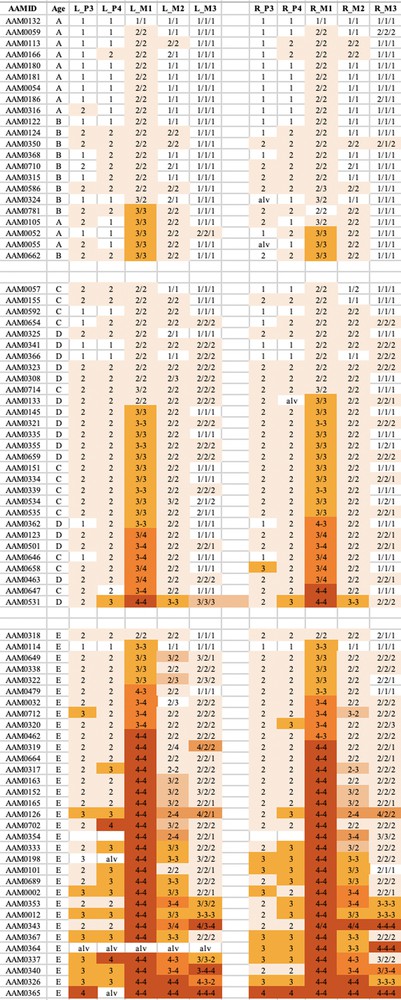
The wear observed in the mandibular teeth of babirusa skulls from Sulawesi. The symbol nomenclature is as in Fig. 3.
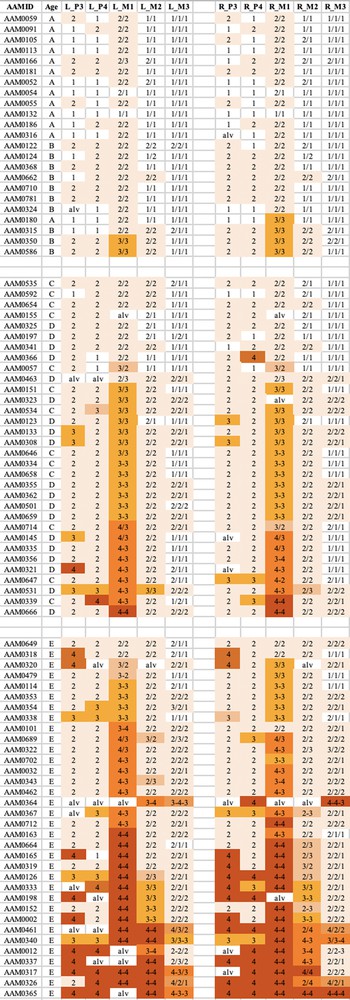
The wear observed in the maxillary teeth of babirusa skulls from Sulawesi. The symbol nomenclature is as in Fig. 3.
Tooth wear was relatively slight in group A + B for both species, with the M1 teeth experiencing most relative wear. There was almost no wear of the M3 teeth.
Tooth wear was then more evident in the C + D group, with the M1 teeth again generally exhibiting most wear. Substantial wear ‘3′ of upper and lower M1 was apparent in this age group, with some skulls showing wear ‘4′, with enamel on part or all of that pillar edge having been worn away.
In group E there was evidence of more widespread wear of the cheek teeth and also increased severity of tooth wear. This was again particularly clear in M1 teeth, but was also apparent among some premolar teeth of the skulls from Buru and the M2 teeth of skulls from Sulawesi. The extent of tooth wear was geographically spread all over Sulawesi, with no regional ‘tooth-wear hot spots’ evident in the data.
4 Discussion
This is the first time that the wear of the cheek teeth of the babirusa has been analysed systematically. It was unsurprising that the skulls of younger babirusa showed less tooth wear than those of older animals. However, the lack of a close link between maxillary canine tooth ‘age’ [17] and the wear exhibited by the cheek teeth was noteworthy. There was no consistent ordering of ‘A’ before ‘B’ or ‘C’ before ‘D’ in cheek teeth wear (Figs. 3–6). Indeed, some of the skulls in the A + B group showed more wear than those in the C + D group, and even some teeth in the A + B group were more worn than those in the ‘E’ group of skulls. Likewise, there was no uniformity of tooth wear within the age groupings. Nevertheless, the similarity of wear on each side of the mouth suggest that the ingested food may go to either side of the mouth for processing and may be passed from one side of the mouth to the other for mastication [18].
The pattern of cheek tooth wear of the babirusa was different from that shown by Sus scrofa [7–10]. The first molar tooth of the babirusa was often the first cheek tooth to experience the most wear, and there was a relative lack of wear, with age, shown by the second and third molar teeth of the babirusa (Figs. 3–6). In Sus scrofa, by way of contrast, there was a relative evenness of progressive wear by the first two molar teeth and a lot more wear of the third molar tooth as that suid was aged. One possible explanation for this may be due to species differences in accessible diet. The large rostral bone of Sus scrofa enables its reinforced rhinarium to dig effectively in hard ground for tough roots to chew and consume [19]. By way of contrast, the babirusa has a soft nose with a thin, partially ossified, cartilaginous plate in its nose, which restricts its probing to wet or sandy soils [1]. It has been recognised for some time that although the babirusa are omnivorous, leaves, and in particular, fruits are important components of their diet [20,21]. The babirusa have been observed on Buru biting soft leaves off low-lying (unidentified) plants and creepers as they walked past them (Macdonald, unpublished). Other food substances reported from Buru and the Sula Islands included the leaves of Alsophyla glauca and Homalomena alba, and the fruits of various fig trees (Ficus spp.), the sweet olot (Hornstedtia rumphii) and the fruit of Rubus fraxinifolius.
Structurally harder foods were also reportedly eaten by babirusa. The seeds of Canarium indicum, Daemonorops robusta and Calamus zollingeri were consumed, as were those of Shorea spp. and Castanopsis buruensis [3,4]. The latter produced a small, energy-rich, hazel-nut sized seed in a very spiky pericarp, which was said to be a preferred food of the babirusa on Sehu and Taliabu Islands. Further research investigations are required to identify the food plants consumed by babirusa in the different geographical regions of Sulawesi.
In zoological collections B. celebensis have been observed cropping the leaves off bramble bushes (Rubus sp.) and low-hanging cherry trees (Prunus sp.) [22] and standing on their hind limbs to browse on the leaves of taller trees [23,24]. A comprehensive list of the plant species that have been reported in the literature as food items of babirusa has been compiled and published [20]. Recent studies have shown that clean food items, free of soil contamination, seem to play a significant role in the diet selection of the babirusa [25]. This is in sharp contrast to the ingestion of the mud from volcanic springs in the northern peninsula of Sulawesi [11,12].
The dynamics of mastication in domestic Sus scrofa have been examined [18,26], and subsequent research has shown the findings to have wider mammalian application [27]. The masseter and temporalis muscles were heavily involved in a co-ordinated way together with the pterygoid muscles. The closure of the jaw was primarily due to the activity of the masseter and pterygoid muscles, on the non-chewing side, and the temporalis muscle on the chewing side. This was followed by the activity of the working side masseter and pterygoid muscles and the non-chewing side temporalis muscle. The masseter muscles increase the crushing force on the food bolus, which was positioned in a slightly lateral position, but they also then contributed to the movement of the bolus over the mandible medially. Recent studies of juvenile Sus scrofa have further demonstrated this ‘yaw’ rotation during occlusion [28] and have shown that it is these muscles, and not the morphologies of the molar teeth, that initiate ‘yaw’ rotation during mastication. The anatomy of the corresponding jaw muscles of the babirusa have been described [29]. The relative lack of wear seen in the babirusa M2 and M3 teeth together with the apparently heavy use of M1 teeth has raised a number of questions concerning the dynamics of mastication in the babirusa. Was it possible that the morphologies of the babirusa's M2 and M3 molars do partially direct the movement of the jaw? Was there more emphasis on the masseters’ food-crushing activity than on ‘yaw’ rotation during occlusion? Was there another diet-specific reason why the M1 teeth are more heavily worn?
The nature of subsequent food digestion may be indicative. The stomach of the babirusa has an elongated shape and a somewhat bulky appearance [30–32]. The total luminal surface area of the adult stomach is 3000 cm2, of which > 70% comprises the ‘honeycomb’ cardiac gland area. Here the mucus-secreting milieu is populated by a complex bacterial microflora of rods and chains of cocci, the digestive functions of which remain to be investigated [31]. This may suggest that the babirusa may carry out more intra-gastric food processing and less oral maceration of food items for digestion when compared to Sus scrofa. Although the sizes of the food particles being swallowed by wild babirusa have never been measured, those seen in the stomachs of zoo housed babirusa have a ‘granular’ appearance (Leus and Macdonald, unpublished).
New methods are being applied to examine the impact of food consumption on the enamel surfaces of teeth [33,34]. Microwear research has investigated the influence of food materials on enamel [35]. Recently, three-dimensional textural analyses of the microwear on the enamel surfaces of molar teeth of four species of extant wild African pigs has enabled conclusions to be drawn on differences in their dietary intake [36]. However, other studies, that examined three-dimensional tooth surface texture analysis in stall-fed and wild boars (Sus scrofa) suggested caution was still required in the formulation of conclusions based on such studies [37]. Silica grit in the form of phytoliths or from exogenous sources (i.e., mud or dust) will abrade enamel in similar ways, but hard food items, such as seeds can also damage enamel [35]. Might the latter be a significant contributor to the wear of M1? Microwear studies were not carried out on these skulls, but some of the photographs were sufficiently detailed to reveal evidence of scratches on the surface of the dentine (Fig. 7).
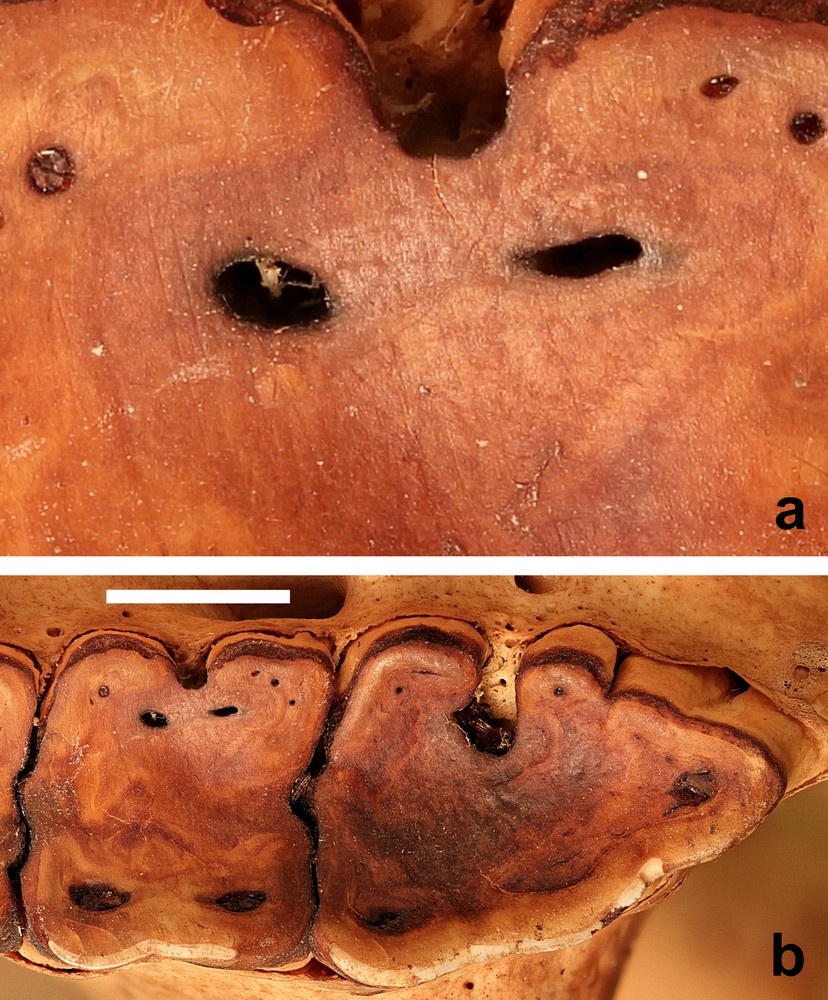
(a) Wear marks on the second molar tooth of (b) the M2 and M3 of the right maxilla (AAM0365) from East-central Sulawesi (maxillary canine tooth age E) (scale = 10 mm).
The similarity in pattern of tooth wear between the babirusa from Sulawesi, where volcanic hot mud ingestion has been reported in the northern peninsula [11,12], and the babirusa from the Sula Islands and Buru, where there have not been any reports of volcanic hot mud ingestion, suggests that the M1 wear is more likely to be plant-diet related. It is also conceivable that the small sizes of phytoliths, which can indeed indent the enamel surfaces, may not have functioned as true abrasives and caused the removal of the enamel from the occlusive surfaces of babirusa M1 teeth [38,39]. An additional factor may be the observation that sufficient contact pressures of materials softer than enamel on an occlusal surface are capable of removing enamel material from it [40]. Wear of enamel occurs when contact pressure is sufficient to break the protein ‘glue’ that holds hydroxyapatite nanorods to the surface.
5 Conclusions
There was close correspondence in wear patterns between each side of the mouth in both species of babirusa and in all age groups. The wear patterns of the mandibular and maxillary teeth, although not identical, were very similar, as were the wear patterns of both babirusa species. Diet selection and subsequent crush-processing of foods were highlighted as potential contributing factors to the heavy wear of M1 teeth and the relative delay in the wear of M2 and M3 teeth. The pattern of cheek tooth wear in male babirusa was not adequate for use to monitor the age of the animal.
Acknowledgements
The author gratefully acknowledges the hospitality shown by the Utomo family, Jakarta during part of this study. The assistance of staff at the Royal Botanical Gardens of Scotland was also much appreciated.
The author would like to thank Friederike Johansson, Göran Nilson and Bianca Ziehmer for their kind hospitality and support during these studies. He would also like to thank the curators and staff of the following museums for access to the babirusa skeletal material that form part of their collections: American Museum of Natural History, New York, USA; Göteborgs naturhistoriska museum, Göteborg, Sweden; Malmö museum, Malmö, Sweden; Museum für Naturkunde, Berlin, Germany; Museum Wallacea, Universitas Haluoleo, Kendari, Indonesia; Museum Zoologicum Bogoriense, Cibinong, Indonesia; National Museum of Natural History, Washington, USA; National Museum of Scotland, Edinburgh, Scotland; Natural History Museum, London, England; Naturalis Biodiversity Center, Leiden, The Netherlands; Naturhistorisches Museum Basel, Switzerland; Naturhistoriska Riksmuseet, Stockholm, Sweden; Naturmuseum Senckenberg, Frankfurt am Main, Germany; Oxford University Museum of Natural History, Oxford, England; Private Collection, Bogor, Indonesia; Private Collection, Buru, Indonesia; Senckenberg Naturhistorische Sammlungen Dresden, Germany; The Field Museum, Chicago, USA; University Museum of Zoology, Cambridge, England; Zoölogisch Museum Amsterdam, The Netherlands; Zoologische Staatssammlung München, Germany; Zoologisk Museum, København, Denmark.
The author would also like to thank Aline Brodin, Edinburgh University Library, for translations into French, and the University of Edinburgh and the Balloch Trust for financial support.


