1 Introduction
Members of the natricid snake genus Trachischium Günther, 1858 are fossorial in nature and are distributed in montane ranges of India, Nepal, Bhutan, China, Myanmar, and Bangladesh [1,2]. The genus currently contains six species: T. fuscum (Blyth, 1854), T. guentheri Boulenger, 1890, T. laeve Peracca, 1904, T. monticola (Cantor, 1839), T. sushantai Raha, Das, Bag, Debnath & Pramanick, 2018 and Trachischium tenuiceps (Blyth, 1854) [2–4]. The snakes of this genus are poorly studied likely due to their fossorial nature; however, these are common elements of high-elevation herpetofaunal assemblages [3,5,6]. The genus was erected based on types of T. rugosum Günther, 1858, now a synonym of T. fuscum, and was placed in the family Colubridae; however, its phylogenetic affinities remained unresolved until recently. Lately, Guo et al. [3,7] showed that Trachischium is sister to a clade including Amphiesma spp., elucidating its deep nested position within Natricidae.
Cantor [8] described T. monticola as a member of the genus Calamaria, as Calamaria monticola, from Naga Hills, now in Nagaland, from northeast India, as a species having 224 ventrals and 44 subcaudals, a bright yellow collar and a whitish dorsal line, the ventral being yellow. This species was subsequently reported from several states in northeastern India and the neighbouring countries [4]. In addition to this, two species Cyclophis rubiventer Jerdon, 1870 from Khasi Hills, Meghalaya and Ablabes albiventer Günther, 1875 from Darjeeling, western Bengal, bearing 15 rows of dorsal scales, were described, which however were synonymised with T. monticola [1,9]. This taxon, nonetheless, appears to be a complex of cryptic species and is in need for detailed taxonomic revision; so are other species of the genus and many fossorial snake species [10].
In the course of a herpetological investigation, we visited Talle Valley Wildlife Sanctuary, Arunachal Pradesh, northeastern India, and collected three specimens of Trachischium sp. that resembled T. monticola in bearing 15 dorsal scale rows, but did differ from it in some aspects, primarily their colouration. In order to ascertain the identity of the specimens from Talle Valley, type material of Trachischium in the collection of the Natural History Museum, London (NHM), as well as non-type material in the collection of Bombay Natural History Society, Mumbai (BNHS) were examined (see list below). Based on our observations, the specimens from Talle valley appear to be a form that is distinct from T. monticola and other congeners, and are herein described as a new species.
2 Material and methods
2.1 Morphology
The species was found during an expedition to Arunachal Pradesh from 25 June 2019 to 5 August 2019, along with other members of the genus. The study was conducted under permit Nos. CWL/Gen/173/2018-19/Pt.V11/2434-43 and CWL/Gen/173/2018-19/Pt.V11/2421-33 issued by the Forest Department of Arunachal Pradesh. Three specimens of the new genus were collected in field with hand, were photographed, and later two specimens were euthanized within 24 h of capture. The other specimen was released after recording scalation and other morphometric data and clipping a single scale for molecular work. The types were euthanized using halothane as per guidelines outlined in Leary et al. [11]. The specimens were fixed in 8% formaldehyde buffer and later stored in 70% ethanol. Liver tissue was collected from the two specimens for molecular work and stored in molecular-grade ethanol prior to fixation. The specimens have been deposited in the collection of the Bombay Natural History Society (BNHS), Mumbai. Measurements were taken with the help of a digital calliper to the nearest 0.1 mm and those for Snout to Vent Length (SVL) and Tail Length (TL) were taken with a string, which was then measured using a scale. Ventral scales (V) were counted as directed by Dowling [12]. Morphological data for the new species were compared with the types of the congeners and their original descriptions too. During the expedition, we also collected tissue from road-killed specimens of the genus for which we have generated molecular data to present a preliminary phylogeny of the genus. Acronyms of institutions where comparative material was examined: NHM - Natural History Museum, London; ZSI - Zoological Survey of India, Kolkata and BNHS - Bombay Natural History Society, Mumbai.
2.2 Comparative material examined
Trachischium fuscum: ZSI 7044 (lectotype), ZSI 7043, from Darjeeling, West Bengal State, India
Trachischium rugosum (= T. fuscum) NHM 1946.1.12.40 (Type) Sikkim, India
Trachischium guentheri NHM 1946.1.12.50 (Syntype) Darjeeling, West Bengal, India
Trachischium quinquelabialis (= T. laeve) NHM 1946.1.12.39 (Type) Kumaon, Uttarakhand, India
Trachischium albiventer (= T. monticola) NHM 1946.1.12.11 (Syntype) Darjeeling, West Bengal, India
T. monticola (n = 10) BNHS 1678 (1 & 2), 1679 (1 & 2), 1681 (1 & 2) Shillong, Meghalaya, India; BNHS 1682 (1 & 2) Tara, Garo Hills, Meghalaya, India; BNHS 1683 (1 & 2) Khasi hills, Meghalaya, India
2.3 Molecular analysis
Genomic DNA was isolated from the preserved tissues of Trachischium spp. using Qiagen DNAeasy kits following protocols proposed by the manufacturer. The molecular methods largely follow Mirza et al. [13] and Mirza and Patel [14]. A fragment of the mitochondrial cytochrome b (cyt b) gene and 16S rRNA gene and nuclear recombination activating 1 (RAG-1) gene were amplified using the primers used by Mirza et al. [13] and Pyron et al. [15]. A 22.4-μL reaction medium was set, containing 10 μL of Thermo Scientific Dream Taq PCR Master Mix, 10 μL of molecular-grade water, 0.2 μL of each 10 μM primer and 2 μL of DNA template, carried out with an Applied Biosystems ProFlex PCR System. The thermocycle profile used for amplification were as follows: 95 °C for 3 min, (denaturation temperature 95 °C for 30 s, annealing temperature 47 °C for cyt b, 58 °C for RAG-1 and 45 °C for 16S for 45 s, elongation temperature 72 °C for 1 min) × 36 cycles, 72 °C for 10 min, hold at 4 °C. The PCR product was cleaned using the QIAquick PCR Purification Kit and sequenced with an Applied Biosystems 3730 DNA Analyzer. Downloaded sequences that also included sequences for nuclear oocyte maturation factor Mos (cmos) were aligned in MegaX [16] using ClustalW [17] with default settings. For optimal partitioning strategy and evolutionary substitution model, aligned data was analysed using PartitionFinder v.1.1.1. [18], implementing a greedy search algorithm under the Akaike Information Criterion (AIC). The Maximum Likelihood (ML) method was implemented to assess phylogenetic relationship with RAxML [19]. Data were subjected to phylogenetic reconstructions with generalised time-reversible (GTR) model as the sequence substitution model, based on the optimal partitioning scheme suggested by PartitionFinder for both ML and Bayesian Inference (BI). Maximum Likelihood analysis was run for 1000 non-parametric bootstrap pseudo-replicates with rapid ML search option. Bayesian Inference was implemented in MyBayes 3.2.2. [20] and was run for 10 million generations and sampled every 1000 generations. BI run included five parallel chains, three hot, and tow cold chains. The standard deviation of the split frequencies of the analysis reached were below 0.01, after which the analysis was not continued further. Twenty-five percent of the trees generated were discarded as burn-in. Uncorrected pairwise p-distance (% sequence divergence) was calculated in Mega X [16] for only 16S with pairwise deletions of missing data and gaps. Divergence was only calculated for 16S as there was paucity of data for cyt b gene. Details of sequences and GenBank® accession numbers are presented in the supporting files (S1).
2.4 Nomenclature acts
The electronic edition of this article conforms to the requirements of the amended International Code of Zoological Nomenclature, and hence the new names contained herein are available under that Code from the electronic edition of this article. This published work and the nomenclatural acts that it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix “http://zoobank.org/”. The LSID for this publication is: urn:lsid:zoobank.org:pub:46A0EB28-E1B0-4E35-B2C8-8D7C4DA45009. The electronic edition of this work was published in a journal with an ISSN, and has been archived and is available from the following digital repositories: PubMed Central, LOCKSS.
3 Systematics
Trachischium apteii sp. nov.
urn:lsid:zoobank.org:act:F26FB189-3898-45D6-84F5-72E083B993B
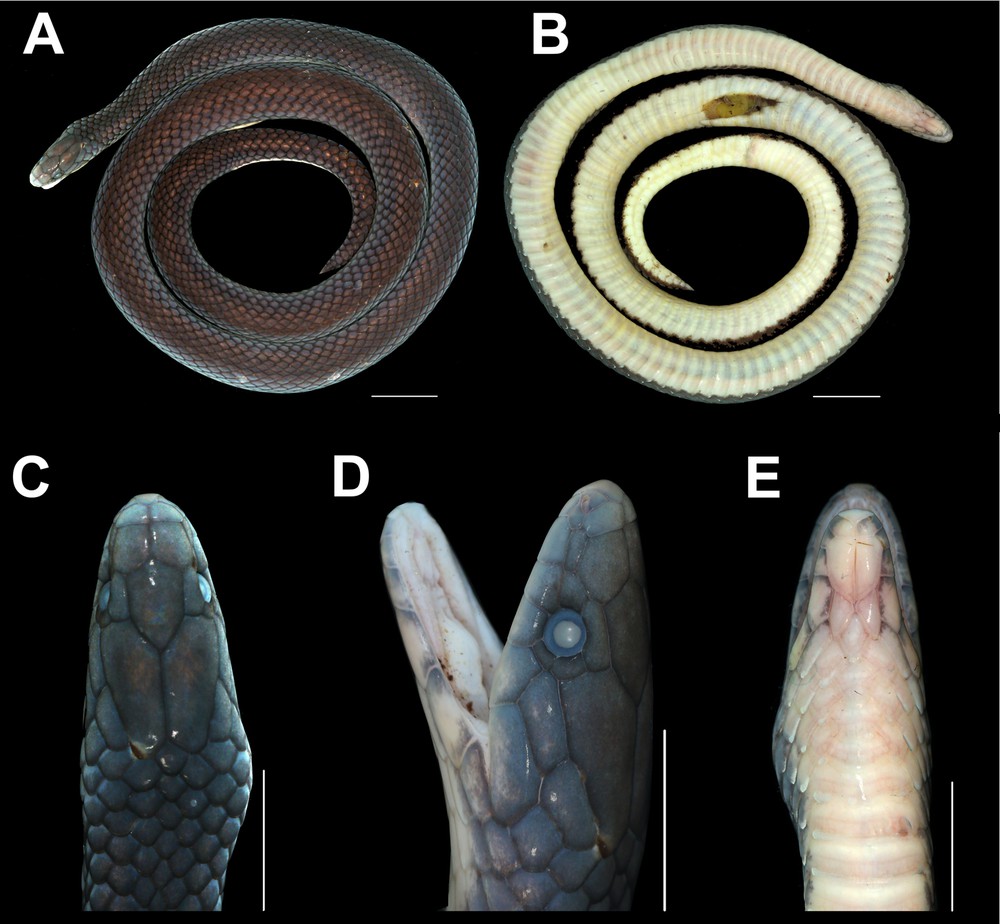
Trachischium apteii sp. nov. BNHS 3550 holotype female (a) body dorsal view (b) body ventral view (c) head dorsal (d) head lateral (e) head ventral. Scale bar: 10 mm (a & b), 5 mm (c–d).

Trachischium apteii sp. nov. holotype female BNHS 3550 in life.
Morphometric and meristic data for type specimens of Trachischium apteii sp. nov.
| BNHS 3550 Holotype | BNHS 3551 Paratype | |
| Sex | ♀ | ♂ |
| SVL | 293.6 | 127.6 |
| TL | 331 | 146.5 |
| tL | 37.4 | 18.9 |
| tL as % of TL | 11.29 | 12.9 |
| w | 7.18 | 3.92 |
| w as % of TL | 2.16 | 2.67 |
| V | 150 | 143 |
| SC | 25 | 27 |
| HL | 9.28 | 6.4 |
| Hw | 5.28 | 3.5 |
| F–Snt | 2.7 | 1.7 |
| Prfl | 1.6 | 1 |
| F–Snt/Prfl | 1.68 | 1.7 |
| FL | 3.3 | 2.65 |
| Fw | 2 | 1.5 |
| PaL | 5.29 | 3.75 |
Holotype: adult female BNHS 3550 from Pange camp, Talle Valley Wildlife Sanctuary, Arunachal Pradesh (27.549322°N, 93.897138°E, elevation 1890 m) collected by a party that included Harshal Bhosale, Gaurang Gowande, Mandar Savant, and Pushkar Phansalkar on 19 June 2019.
Paratype: juvenile male, BNHS 3551, collected on 16 June 2019, same data as the holotype.
Diagnosis: Large-sized member of the genus, reaching SVL of 293–299 mm. Tail comprising of 11–13% of total length. Dorsal scales smooth and glossy, arranged in 15 rows throughout the body. Six supralabials, third and fourth in contact with orbit. Temporals 1 + 1, anterior temporal long. Ventrals 143–150, subcaudals 25–28. Dorsum colouration dark brown to black with faint dorsal longitudinal lines, lacking yellow patch on the neck and the belly being yellowish.
Genetic divergence: Intraspecific divergence (uncorrected p-distance) observed for the gene 16S is 0% (n = 3). Interspecific divergence with congeners ranges from 1–4% (Table 2).
Uncorrected pairwise p-distance for the 16S rRNA gene for the Trachischium species (in %).
| Trachischium apteii sp. nov. | |
| T. apteii sp. nov. (n = 3, Talle Valley Wildlife Sacntuary, Arunachal Pradesh) | 0 |
| T. cf. guentheri (Eagleneast Wildlife Sanctuary, Arunachal Pradesh) | 3.1 |
| T. cf. tenuiceps (Eagleneast Wildlife Sanctuary, Arunachal Pradesh) | 2.4 |
| T. cf. guentheri (Shergaon, Arunachal Pradesh) | 1 |
Comparison: A Trachischium resembling T. monticola (and its putative junior synonyms, detailed comparison provided below), differs from T. fuscum, T. guentheri, T. laeve, and T. tenuiceps in possessing 15 dorsal scale rows instead of 13 at mid-body. The new species differs from T. monticola and its synonyms, in possessing higher ventral count 143–150 (versus 113–125 in T. monticola, T. rubiventer & T. albiventer), very faint dorsal longitudinal lines, lacking yellow patch on the neck and the belly being yellowish (versus distinct longitudinal light and dark stripes throughout with a clear yellow patch on the neck with a red belly T. monticola & T. rubiventer).
Description of female holotype BNHS 3550 (Fig. 1). Some morphometric and meristic data are given in Table 1. Specimen in good condition with a mid-ventral incision from 90th ventral to 97th ventral scale. Body preserved in a flat coil with the head resting outside the coil. Colours have not noticeably faded (Fig. 1A–B). Morphometric data of a few characters of the types is presented in Table 1.
Head short, 9.28 mm, with slightly elongate, sub-conical snout in lateral view. Snout gradually tapering to blunt, rounded tip in dorsal view (Fig. 1C). Rostral subtriangular, longer than wide (0.99 mm long, 0.75 mm wide). Nasals appear undivided, bi-lobed with a horizontal nostril in its anterior lobe. Left and right nasals not in contact, separated by the intervening rostral scale (Fig. 1D). Paired internasals large, longer (1.2 mm) than wide (0.72 mm), much larger than nasals and rostral, though smaller than the prefrontals (Fig. 1C). Prefrontals slightly longer (1.80 mm) than wide (1.75 mm), covering a greater area of the snout, lateral wing of the prefrontals extending to the lateral aspect of the head.
Six supralabials, third and fourth entering orbit. First supralabial very small and, apart from second supralabial, contacts only rostral and nasal. Second supralabial twice the size of the first supralabial, thin strip contacting nasal, loreal, and first and third supralabials. Third and fourth supralabial scales much larger, longer than high. Fifth supralabial in contact with postocular, anterior temporal, and fourth and sixth supralabial. The sixth supralabial is the largest. Single elongate loreal and loosely pentagonal preocular. Shield-shaped frontal notably longer (3.3 mm) than broad (2 mm), and about half as long as the paired parietals. Temporals 1 + 1, subequal in size; the anterior one inserts deeply between last two supralabials. Supraocular slightly larger than the pair of postoculars (Fig. 1D).
Anterior of lower jaw dominated by large pair of anterior genials meeting along midline mental groove, prevented from reaching margin of mouth by small mental and three very thin infralabials. Mental short, broad, with tripartite anterior end (Fig. 1E). Anterior two infralabials short and thin, the second marginally larger. First two infralabials together shorter than long, narrow third, and in lateral view falling notably short of halfway along the length of the anterior genials. Fourth and fifth infralabials much larger, about the same size as each pair of posterior genials. First unpaired mid-ventral scale immediately behind posterior genials, with approximately equidistant transverse and longitudinal axes. The second ventral scale is the first that is wider than long.
Body subcylindrical, ventral surface a little flattened. Dorsal scales in 15 rows, up to posterior-most ventral. Dorsal scales imbricate, regularly arranged, evenly sized. All body scales macroscopically smooth and glossy, lacking keels. Ventral scales 150 (V) in number + three preventrals. Anal shield divided, similar in size to the last ventral scale. Subcaudals paired, 25 in number. Tail terminating in a tapering apical spine. Total length 331 mm, tail length 37.4 mm, tail/total length ratio 0.11. Thirteen dorsal scale rows at the beginning of the tail, reducing to about six at mid tail, three surrounding the base of the terminal spine.
Body and scales iridescent throughout. Overall, in a shade of greyish black, head shields in a shade of dark grey with paler edge (Fig. 1A).
Variation observed. The male paratype BNHS 3551 agrees with the holotype in most regards except for the details noted here: the paratype bears two very faint longitudinal stripes on the dorsum, running from the temporal region up to the cloaca, more evident in life. The stripes appear faded in preservative. The male bears a slightly longer tail than the female in terms of proportions, as well as it has a few more subcaudal scale than the holotype. Other morphometric details listed in Table 1. The uncollected individual measured SVL 299 mm and tL 43 mm and bore 147 ventrals and 28 subcaudals.
Etymology: The specific epithet is a patronym, honouring Dr. Deepak Apte, Director of the Bombay Natural History Society, marine biologist and conservationist for his contribution to conservation, and for his continued support to HB and to the Arunachal Expedition, which led to the discovery of the new species.
Natural history notes: The holotype, paratype, and another uncollected individual were found under logs during a daytime search. The individuals were likely seeking refuge under the logs during the day. Locals informed the scientific team that the snakes have also been found while digging during non-monsoon seasons.
4 Discussion
The genus Trachischium is recovered as clade embedded within Natricidae and is sister to the genera Herpetoreas and Hebius [3,7,21]. The results from phylogenetic reconstruction recovered in the present work are congruent with previous studies concerning the generic relationship [3,21]. However, the phylogenetic relationship within the genus remains unresolved as the current study suffers from incomplete sampling in terms of species and molecular markers. The results of our phylogenetic analysis show that the Indian specimens form a well-supported clade (ML bootstrap 96, BI posterior probability 0.98) and Trachischium cf. monticola from China is basal to this clade (Fig. 3). Support for relationships within the Indian clade are too poor to make any conclusive inference. However, based on morphology, the new species, T. apteii sp. nov., appears to be allied to T. monticola and its synonyms in bearing 15 dorsal scale rows, but differs from it by its high ventral count and dorsal coloration. The type locality of Trachischium monticola lies south of the Brahmaputra River, whereas the type locality of the new species is located north of the river. The Brahmaputra River has been shown to be a biogeographic barrier for gene flow [22–24]. Fresh specimens from type localities of all species of the genus Trachischium – especially, their putative synonyms of all species – must be collected for a systematic review. The examination of museum material and genetic data in the present study, attested by the discovery of T. sushantai, hint on the presence of undocumented diversity within the genus.

Maximum Likelihood molecular phylogeny of selected members of natricine snakes showing the phylogenetic position of the genus Trachischium reconstructed from concatenated 2980 bp of cmos, 16S, cyt b, RAG1. The analysis was run with GTR as the sequence substitution model and was simulated for 1000 non-parametric bootstrap pseudo-replicates with a rapid ML search. Numbers at nodes represent ML bootstrap support followed by BI posterior probability. The outgroup Aspidura ceylonensis was removed for representational purpose.
As a description of the new species of Trachischium, a keelback [25] and a pit viper [26], coupled with the existing ophidian diversity [4,5,27] only highlight the need for dedicated surveys across Arunachal Pradesh. Anthropogenic pressures like road widening, construction or dams and hydropower plants threaten the forests and biodiversity across Arunachal Pradesh.
Acknowledgments
The authors thank the Forest Department of Arunachal Pradesh for issuing necessary permits (permit Nos. CWL/Gen/173/2018-19/Pt.V11/2434-43 to ZM and CWL/Gen/173/2018-19/Pt.V11/2421-33 to GG) to conduct surveys across the state. Tulika Kedia (Singinawa Conservation Foundation) supported ZAM and Shripad Halbe (Brihad Bharatiya Samaj) supported HB and his team. The authors also thank Rahul Khot BNHS for his constant support. GG was supported by the Rufford Small Grants for Nature Conservation. ZAM acknowledges The Newby Trust Limited for a grant to travel to the Natural History Museum, London. GG is indebted to Principal and HoD, Biotechnology, Fergusson College, and Principal, Ankur Patwardhan, HoD, and his advisor Dhanashree Paranjpe, Ramalingaswami Fellow, Biodiversity, Abasaheb Garware College, for their constant support and encouragement. The authors are extremely thankful to Pushkar Phansalkar and Mandar Savant for their help and support on the field (#TheArunachalExpidition). Fieldwork at Pange would not have been possible without the help of Jayanto, Sardar, Habung, Yapa and Thado. ZM thanks K. Vijay Raghavan for guidance and all the lab mates for their support.


