1. Introduction
There are an estimated 100 billion neurons in the adult human brain, 70 million in the adult mouse, 10 million in the adult zebrafish [1, 2]. Brain neurons control most body activities, including sensory, motor, autonomic, emotional and executive functions. How were they generated, and when? Are there common rules?
The generation of neurons, referred to as neurogenesis, is a multistep and gradual process that originates from a neural progenitor cell and ends with the formation of a mature cell capable of network communication, the neuron. Along the way will occur cell divisions, commitment to a neuronal fate, entry into the postmitotic state, detachment and migration away from the progenitor territory, and the acquisition of neuronal characteristics such as electrical excitability, neurotransmitter(s) synthesis, an elaborated polar morphology including axon and dendrites, and connectivity with other cells (Figure 1A). The sequential order of these steps is not fixed, and the neurons generated are highly diverse, in identity, morphology, plasticity, circuit and function. Increasing this diversity, neurogenesis is a lifelong process, taking place from embryo to adult. As such, acquiring a comprehensive understanding of neurogenesis regulation is an immense task (for recent reviews, see [3, 4, 5]).
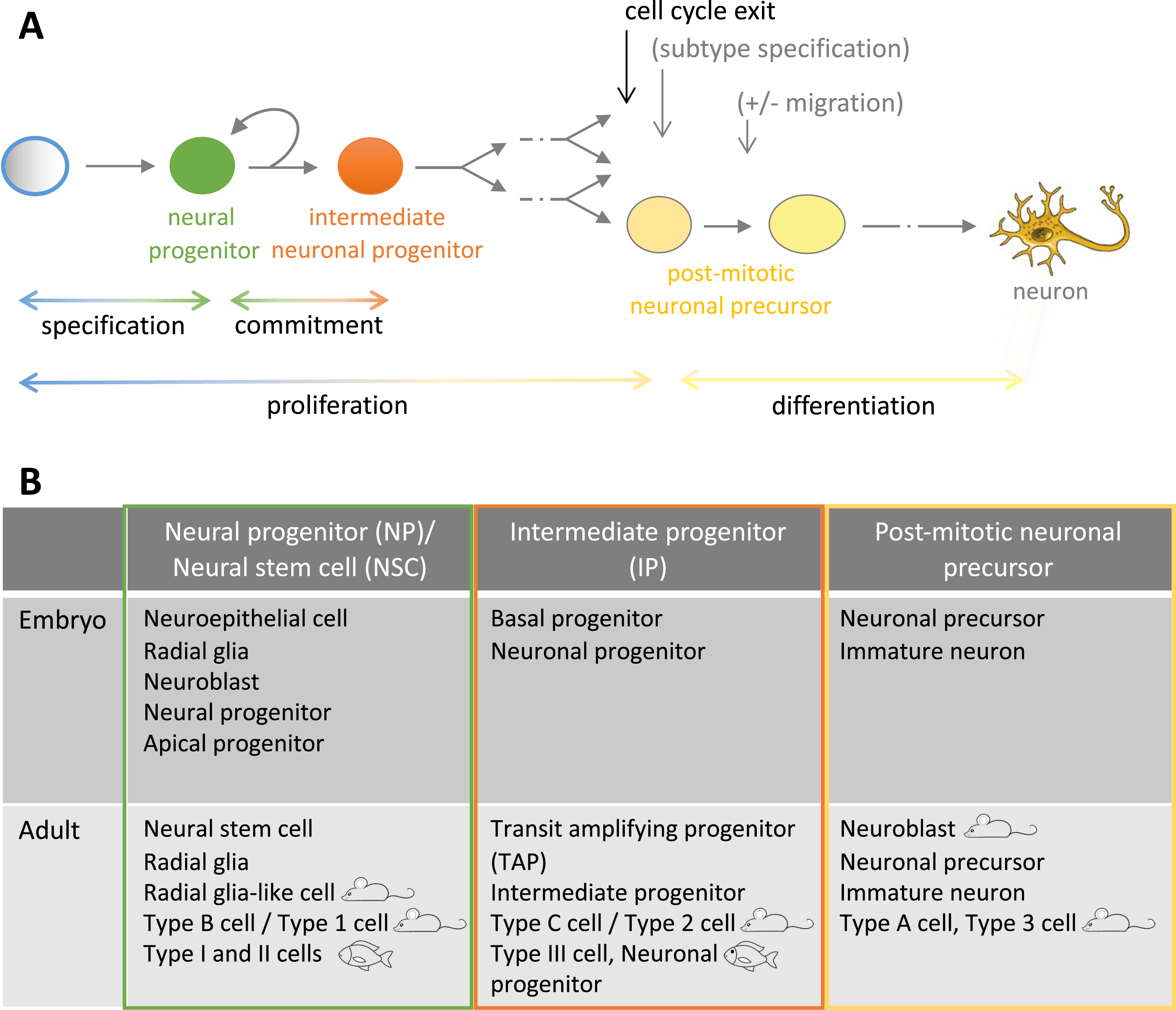
Neurogenesis steps and nomenclature. (A) Progression of neural progenitors along the neurogenic lineage (from left to right). The relative order of cell cycle exit and neuronal subtype specification is not fixed. (B) Cell types encompassed by the nomenclature NP/NSCs, IPs and precursors in the embryonic vs. adult brain and in mouse vs. zebrafish.
The present review will focus on the early steps of the neurogenesis process, which cover the transition from a progenitor to the post-mitotic and committed neuronal precursor. These initial events largely control the location, timing and extent of neuronal generation. As a driving thread, this review is also a comparative analysis of embryonic and adult neurogenesis processes, with focus on Notch signaling, the major regulatory pathway controlling neurogenesis during a lifetime [4, 6]. This is because general principles are better extracted from comparisons, and also because current translational research places high hopes in the manipulation of neural progenitors or in vitro models of embryonic characteristics to understand or ameliorate adult pathologies of neurons loss. Finally, it will largely make use of knowledge gained from the teleost fish model Danio rerio (zebrafish) which, among other practicalities, offers the unequalled possibility to film neurogenesis “in situ” in vivo in both the embryonic and adult brain under fully non-invasive conditions, thanks to the transparency of its embryos and of some pigmentation-deficient adults [7, 8, 9, 10, 11]. We will, of course, refer to other models, in particular the mouse, when the latter led to the princeps discovery or when interspecies comparisons add to the extraction of principles. As much as extracting shared and divergent neurogenesis principles between the embryonic and adult brain, we will aim to identify remaining key questions in the field.
2. Neural progenitor cells
2.1. Definitions
There is extensive work, associated with diverse and sometimes subtle nomenclature differences, to characterize the progenitors at the origin of neurons in diverse brain locations, time points or species. For clarity, we will adopt a simple rule here, which essentially reflects time, during life or along lineage progression. This nomenclature is placed alongside others in Figure 1B. In sum, we will refer to neural progenitors (NPs) when cells can generate both neurons and glial cells and their long-term neuron-generation potential is either limited (typically a few weeks in a vertebrate model species such as zebrafish or mouse) or unknown, to neural stem cells (NSCs) when cells can generate both neurons and glial cells but their long-term potential is extensive (typically a few months or more), and to intermediate neuronal progenitors (IPs) for the proliferating progeny of NPs or NSCs, that will exclusively generate neurons and will exhaust at short term. With this nomenclature, neurogenesis occurs in the order NP > IP > neuron or NSC > IP > neuron, and typically, NSCs are an adult cell type, while NPs refer to neural progenitors in the embryo. Indeed, even if some NPs have been shown to give rise to adult NSCs and are therefore long-lived (see below), not all of them do, and it is generally impossible to distinguish between these fates at the time of observation.
2.2. Neuroepithelial cells and radial glia
2.2.1. Neural progenitors of the embryonic neural tube
Neural progenitors (NPs) of the embryonic neural tube are apico-basally polarized cells arranged in a monolayer bordering the neural tube lumen (ventricle) (Figure 2A,B) (reviewed in [12, 13]). Their apical membrane, in contact with the cerebrospinal fluid, is characterized by several specialized structures, such as a primary cilium pointing into the lumen, a Cadherin ring, and junctional complexes [14, 15, 16, 17]. The latter include tight junctions marked by the Zona Occludens 1 (ZO1) protein that connect with adjacent progenitors at their apico-basal interface and seal the neural tube lumen. Their basolateral membrane is elongated and connects with the pial surface of the neural tube. At early developmental stages (tail bud to a few somite-stage in zebrafish, E7-E8 in mouse), neural progenitors are neuroepithelial cells (NE) (Figure 2A), readily specified from the neuroectoderm. They express transcription factors that sign both their neural and their progenitor states, such as Sox2 or Hairy/Enhancer-of-Split (Hes/Her) family members [18, 19, 20]. As neurons become generated and the neural tube thickens, the basolateral membrane of progenitors elongates. The acquired radial morphology is associated with the expression of astroglial markers in addition to progenitor markers, hence the name of radial glia (RG) (Figure 2B). These markers include intermediate filaments (Vimentin; Glial fibrillary acidic protein, GFAP) and the Brain lipid binding protein (BLBP). Both NE and RG cells are actively dividing and exhibit the characteristic feature of interkinetic nuclear migration (INM), where the nucleus transits from apical to basal positions and back within the cell body as a function of cell cycle phases [12, 21]. Cytokinesis events occur apically along the ventricular plane, and INM is believed to avoid steric hindrance in this location for progenitor division to take place. Embryonic NE and RG are very similar between species, although, compared to mouse, zebrafish NE polarize relatively late and express glial markers almost from their onset [22], the transition from NE to RG is therefore blurred.
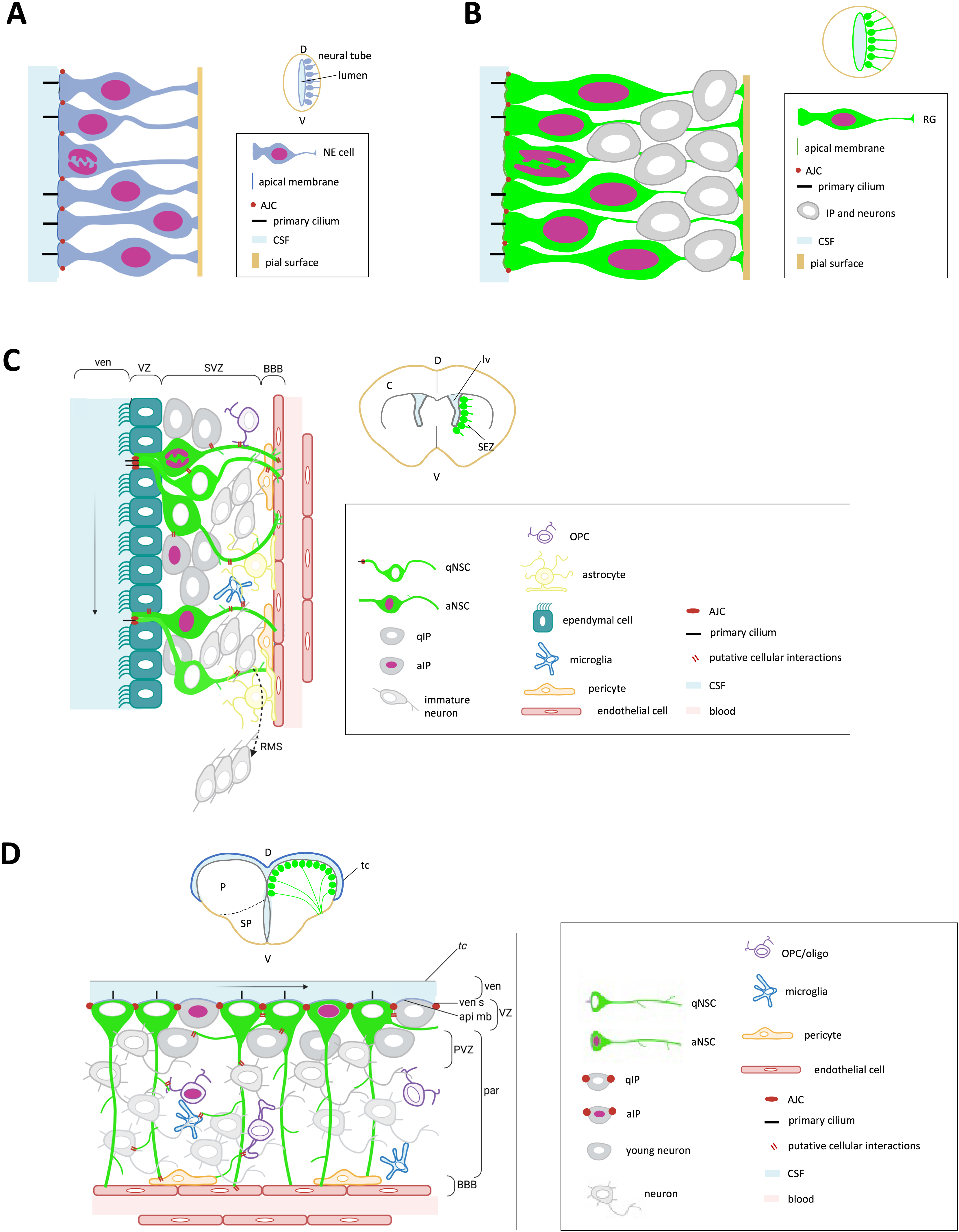
Morphology of NPs/NSCs and architecture of the neurogenic niches in the embryonic vs. adult brain and in mouse vs. zebrafish. (A–D) Schematic high magnification views of the cytoarchitecture of the ventricular zone when observed in cross sections (main panels), whole forebrain in cross-sections (small panels), and legends for colors and structures (boxed). (A,B) NPs at early embryonic stages (A) are neuroepithelial progenitors (NE), they transform into radial glia (RG) at later embryonic stages (B). (C,D) NSCs in the subependymal zone of the mouse adult brain (C) and in the zebrafish pallium (D) are RGs. Abbreviations: a, activated; AJC, apical junction complex; api mb, apical membrane; BBB, blood–brain barrier; C, cortex; CSF, cerebrospinal fluid; D, dorsal; IP, intermediate progenitor; lv, lateral ventricle; NE: neuroepithelial cell; OPS, oligodendrocyte progenitor cell; P, pallium; par, parenchyma; PVZ, periventricular zone; q, quiescent; RG: radial glia; RMS, rostral migratory stream; SEZ, subependymal zone; SP, subpallium; SVZ, subventricular zone; tc, tela choroida; V, ventral; ven, ventricle; ven s, ventricular surface; VZ, ventricular zone. Masquer
Morphology of NPs/NSCs and architecture of the neurogenic niches in the embryonic vs. adult brain and in mouse vs. zebrafish. (A–D) Schematic high magnification views of the cytoarchitecture of the ventricular zone when observed in cross sections (main panels), whole forebrain in ... Lire la suite
2.2.2. Neural stem cells of the adult brain
Niches of constitutive neurogenesis have been identified in the adult brain in all vertebrates studied to date (with ongoing controversies for the human brain), although major differences exist in their location and extent, activity, and lifetime. For example, these niches are restricted to the forebrain in mouse, with three major sites described that are especially active in the young adult: the ventricular-subventricular zone of the lateral ventricle (vSVZ, also called sub-ependymal zone, SEZ) (Figure 2C), the sub-granular zone of the dentate gyrus of the hippocampus (SGZ), and the ventricular wall of the hypothalamus [23, 24, 25]. In contrast, neurogenic niches exist in all brain subdivisions in the adult zebrafish as well as in the retina and are active until a comparably late age (considering the fact that the two species have the same lifespan) [26, 27, 28]. A major niche that has been extensively studied is located in the pallium (dorsal telencephalon) (Figure 2D). We will restrict the following discussion to the forebrain.
The majority of NSCs across species are radial astroglia, frequently referred to as RG-like cells [29]. In most niches except the SGZ, which is not ventricular, they maintain an apical contact with the brain ventricle and cerebrospinal fluid. During interphase, this apical membrane bears a primary cilium. The RG basal process contacts the pial brain surface (in the case of small-sized brain territories) or blood vessels at specific interfaces contributing to the blood–brain barrier yet permitting systemic communication to NSCs [7, 30]. This basal process is generally extensive and highly branched sub-apically or deeper into the parenchyma. Non-radial astroglial NSCs also coexist with radial NSCs in the SGZ, in slightly lower proportions [31, 32]. Finally, like in the embryo, NE-like cells with long-lasting neurogenic capacity, hence NSCs, have also been described in the adult zebrafish brain. These different NSC subtypes share expression of generic progenitor markers (e.g., Sox2) with variations for others [33, 34]. Astroglial markers, such as cytoskeletal elements (Glial Fibrillary Acidic Protein, GFAP; Nestin; S100beta in zebrafish), Fatty Acid Binding Proteins (e.g., Brain Lipid Binding Protein, BLBP), or metabolic enzymes (e.g., Glutamine Synthase, GS) also characterize RG-like and non-radial glial NSCs, with some regional differences [35]. It is important to note, however, that these markers are often shared between physiologically neurogenic vs. non-neurogenic cells types (e.g., between RG-like cells and astrocytes in mouse) and should be associated with functional assays assessing self-renewal and neurogenesis potential, at least at the population level, to be conclusive.
2.2.3. Markers and properties
The overlaps and specificities of markers of NPs, NSCs and NEs are listed in Figure 3. The glial markers expressed by embryonic RGs encode factors relevant for known astroglial functions, such as glutamate synthesis and metabolism. This suggests that embryonic RGs may exert such roles in addition to their progenitor properties. This point becomes very relevant when considering adult NSCs, as a major functional difference is observed in adult forebrain astroglia between zebrafish and mouse. The zebrafish adult forebrain is devoid of parenchymal astrocytes [36, 37]. As a counterpart, radial glia NSCs do express markers of mature astroglia, suggesting that they also are endowed with the dual properties of progenitor cells and astroglia [38]. In contrast, these functions are split between NSCs and parenchymal astrocytes, respectively, in the adult mouse forebrain. Hence, zebrafish NSCs appear multifunctional while mouse NSCs are specialized stem cells. It should be noted that several observations argue against the fact that zebrafish RG NSCs would simply correspond to RGs at an intermediate state of maturation, which would not have chosen yet between the NSC and astrocytic fates. First, adult zebrafish RG do exhibit adult-specific features such as a long quiescence phase (see below). Second, their markers of astrocytic functions are not or lowly expressed in RG at juvenile stages, attesting to a maturation since that stage [39]. Third, the choice towards an astrocytic fate in fact occurs at a relatively early stage of mouse embryonic development [40, 41]. Extramural parenchymal astrocytes in the adult mouse striatum can however be recruited for neurogenesis following stroke or lesion, showing that astrocytes have conserved some neurogenic capacity although not under physiological conditions [42, 43, 44].
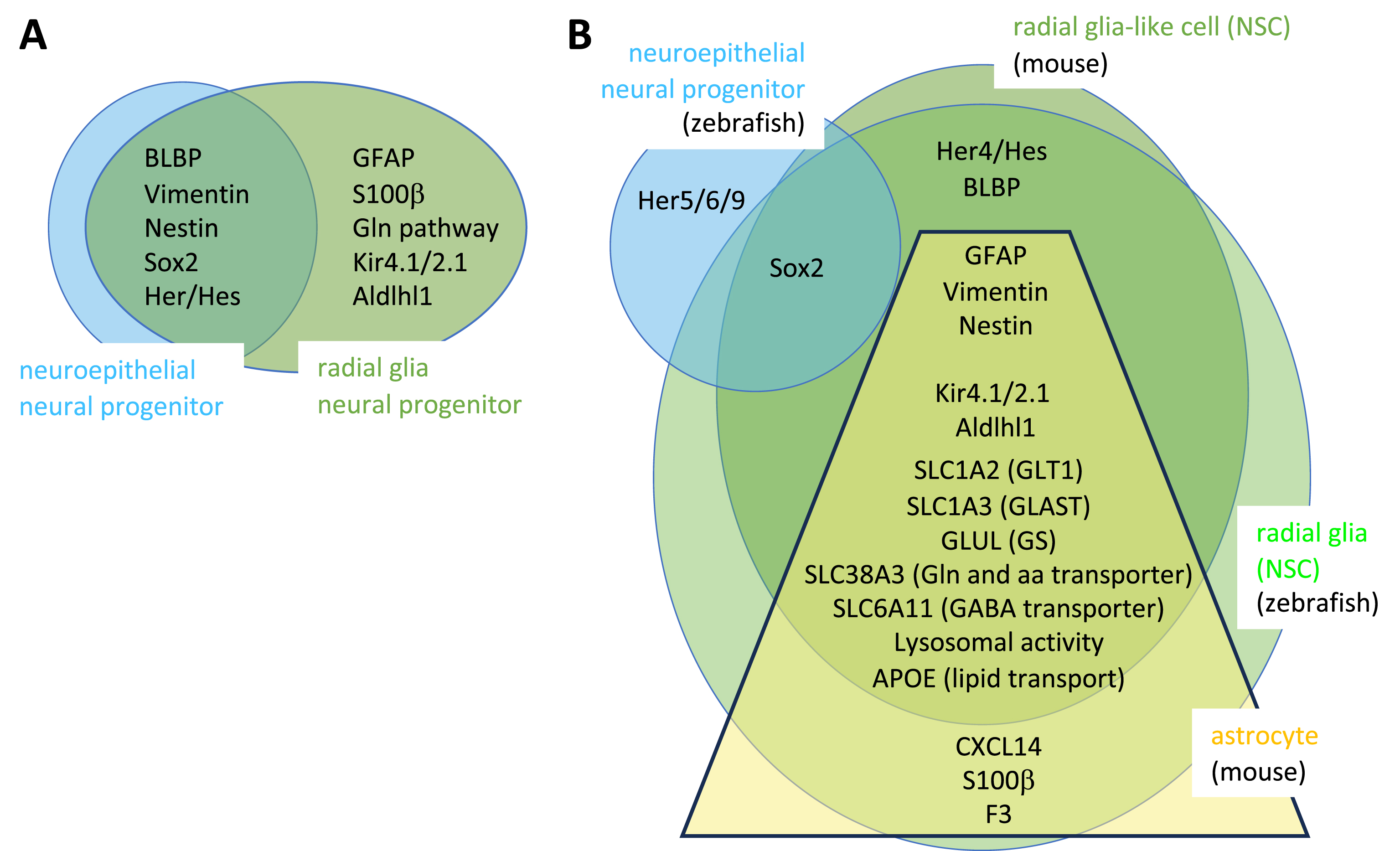
Radial glia markers in the embryonic and adult brain compared to other progenitors and astroglia in zebrafish and mouse. (A–B) Distribution of marker proteins between the different neuroepithelial and astroglial cell types in the embryo (A) and adult (B) in zebrafish and mouse (no species indicated when the markers are shared in both species). In B, the yellow trapeze including astrocyte genes in mouse reflects the expression gradient (low expression to high expression from top to bottom) of the encoded factors between radial glia-like cells (bona fide NSCs) and astrocytes (which are not neurogenic under physiological conditions). Masquer
Radial glia markers in the embryonic and adult brain compared to other progenitors and astroglia in zebrafish and mouse. (A–B) Distribution of marker proteins between the different neuroepithelial and astroglial cell types in the embryo (A) and adult (B) in ... Lire la suite
2.3. Take home message
The characterization of neural progenitor subtypes and their comparison in the embryonic and adult brain lead to a refined understanding of the NE to RG transition. NE and RG progenitors co-exist in the embryonic brain, as this transition affects most, but not all, NEs. They also co-exist at least in the zebrafish brain where they can still be observed at adulthood. Instead of RG, the adult mouse brain hosts specialized NSCs (RG-like cells) and parenchymal astrocytes. Counterintuitively, zebrafish RG NSCs express markers of mature astroglial function, which are the attribute of astrocytes but not RG-like NSCs in mouse.
3. Lineages and niches
Genetic tracing studies indicate that adult NSCs originate from embryonic NPs, as opposed to, for example, the dedifferentiation of mature neurons or glial cells in the adult brain. However, while embryonic NPs distribute broadly along the neural tube ventricle, in a pattern similar between vertebrate embryos in different species, adult NSCs are restricted to niches and display a species-specific distribution, much broader in zebrafish than in mouse. Furthermore, at both embryonic and adult stages, NE and RG cells occupy selective and exclusive locations. How are these patterns generated and their temporal evolution controlled between embryo and adult stages?
3.1. A (constitutively) neurogenic lineage from radial glia
Embryonic RG give rise to adult RG NSCs in the adult forebrain of mouse and zebrafish (Figure 4). Specific embryonic RG have been traced genetically using Cre-lox recombination, for example using as drivers the Gli1, Hopx or Nestin promoters for SGZ precursors [45, 46, 47, 48], promoters such as Gli1, Hopx (from post-natal day 1 onwards), Gfap or Nestin for SEZ precursors [45, 49, 50, 51, 52, 53], and the her4 promoter for RG of the zebrafish adult pallium [33]. The question of whether embryonic RG NPs maintain, or not, a sustained neurogenic activity during the transition from embryo to juvenile to adult has been addressed recently. The use of conditional recombination in zebrafish, together with the fact that the neurons generated in the pallium over a lifetime neither migrate nor die at a detectable rate, made it possible to observe that neuronal clones issued from individual embryonic RG can expand continuously across the pallial parenchyme. These results demonstrate the existence of RGs that maintain her4 expression from embryo to adult and are continuously neurogenic, showing that a continuous neurogenic lineage ensures the transition from embryonic RG NPs to adult RGL NSCs [33, 54]. The same conclusion was later reached in the mouse dentate gyrus for SGZ NSCs [50]. The SEZ appears to contrast with this situation, as embryonic RG along the 4th ventricle and destined to populate the SEZ enter quiescence at an early stage [51, 52, 55]. Whether this reflects a bona fide interruption of neurogenesis between embryonic stages and adulthood, a heterochronic quiescence entry between brain territories, or an RG behavior that may have been missed in other brain territories, remains to be assessed precisely.
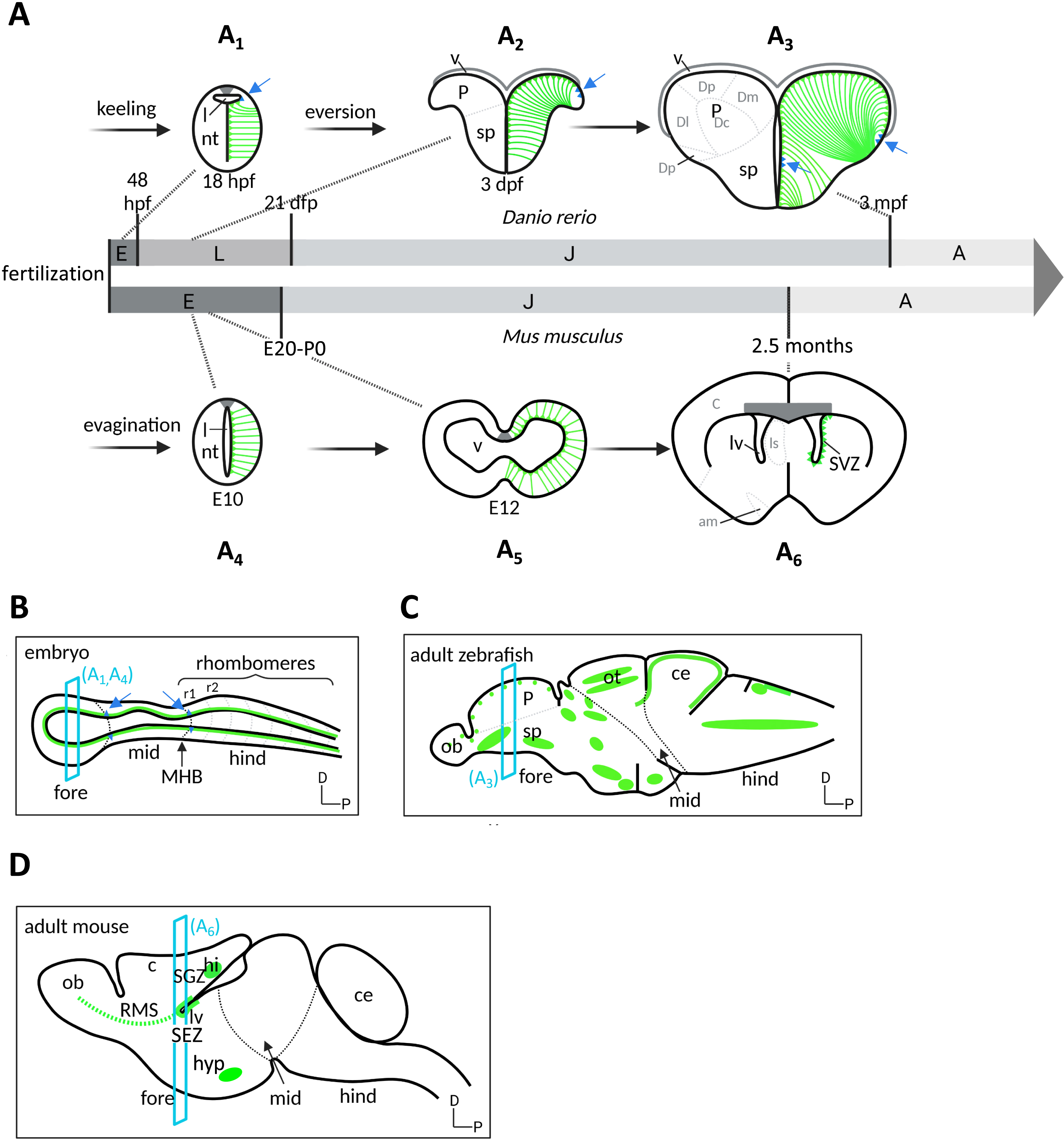
Embryonic to adult neurogenesis in zebrafish and mouse. (A) Temporal progression of neuroepithelial progenitors (NEs, blue), and radial glia NPs and NSCs (green), represented on schematic cross-sections (dorsal up) of the forebrain of zebrafish (A1–A3, top) and mouse (A4–A6, bottom) over a lifetime. Temporal nomenclature: E, embryo; L, larva; J, juvenile; A, adult; hpf, hours post-fertilization; dpf, days post-fertilization; E, embryonic day post-fertilization. In the zebrafish telencephalon, the formation of an initially compact neural rod (process referred to as “keeling”) is then followed by the lateral expansion of the dorsal neural territories and tela choroida (process referred to as “eversion”). In contrast, evagination takes place in mouse to create the central canal. (B–D) Location of neurogenic niches (RG, green; NE: blue triangles, also indicated by blue arrows) on schematic sagittal sections of the brain in a prototypical mouse or zebrafish embryo at mid-embryogenesis (B) and in the zebrafish (C) and mouse (D) adult brains (anterior left, dorsal up). Section planes (blue) correspond to the cross-sections in A. Territorial nomenclature: am, amygdala; c, cortex; ce: cerebellum; Dc, central pallium; Dd, dorsal pallium; Dl, lateral pallium; Dm, medial pallium; Dp, posterior pallium; hi, hippocampus; hyp, hypothalamus; l, lumen; lv, lateral ventricle; mid: midbrain; MHB: midbrain–hindbrain boundary; fore: forebrain; hind: hindbrain; nt, neural tube; P, pallium; ob: olfactory bulb; ot: optic tectum; r1/2, rhombomeres 1 and 2; sp, subpallium; RMS, rostral migratory stream; SEZ, subependymal zone; SGZ, subgranular zone; v, ventricle. Masquer
Embryonic to adult neurogenesis in zebrafish and mouse. (A) Temporal progression of neuroepithelial progenitors (NEs, blue), and radial glia NPs and NSCs (green), represented on schematic cross-sections (dorsal up) of the forebrain of zebrafish (A1–A3, top) and mouse (A4–A6, bottom) ... Lire la suite
3.2. An embryonic to adult NE lineage at the origin of radial glia, neurons and growth
NE populations are positioned at the boundary between neural tube subdivisions at mid- to late embryogenesis (Figure 4B) [56]. A few of them have been reliably traced in the zebrafish using genetic Cre-lox labeling, and shown to contribute to the persisting NE NSC populations identified in the juvenile or adult zebrafish brain. For example, the small her9/her6-positive NE population located on the dorsal midline at the tel-diencephalic junction in the 24-h embryo contributes to the NE population found along the postero-lateral edge of the adult pallium (Figure 4C) [33]. Considering the morphogenetic eversion of the pallium taking place in teleosts [57], this location in the adult telencephalon does indeed correspond to the remnants of the dorsal midline, at the junction with the choroid plexus. In the adult pallium, this NE pool expresses her9, is highly proliferative, generates neurons and more of itself by amplification as well as neurogenic RG NSCs that leave the NE zone to contribute to the progressive enlargement of the RG population of the pallial ventricle [33]. A similar scenario was described for her5-positive NE progenitors at the midbrain–hindbrain boundary: embryonic NE progenitors located at the MHB contribute to the NE NSC pool at the adult MHB, which generates neurons and neurogenic RG NSCs that settle away from the NE pool in an age-dependent order [34]. As such, embryonic and adult NEs are lineage related, and play multiple functions: they are neurogenic lifelong generators of RG NSCs, and amplification centers that act as growth zones. While NE pools are also found in the mouse embryonic neural tube [58], their adult equivalents have not been analyzed as such. The cortical hem, however, is in a homologous location to the tel-diencephalic dorsal NE pool in zebrafish, and contributes neurons and scaffold glial cells to the hippocampus [5, 59]. Whether a NE remnant can also be found in this location has not been studied.
3.3. Sculpting NP and NSC niches
In the embryonic neural tube, RG and NE NP populations occupy alternating domains along the antero-posterior axis (and along the dorso-ventral axis in the hindbrain and spinal cord), with RG pools located in the center of neural tube subdivisions while NE pools are located at neural tube boundaries [56]. This organization corresponds to an alternation between actively neurogenic (RG) domains and domains of delayed neurogenesis (NEs) which in fact already pre-exists in the very early neural plate, prior to the NE > RG transition that affects most NEs at mid-embryogenesis and is prefigured in the expression domains of her/Hes genes. As such, NP activity becomes patterned according to the general vertebrate body plan, contributing to the generation of the segmented embryonic neuronal scaffold of the larva, which controls early larval functions.
As discussed above, the lineage relation between embryonic and adult RGs on the one hand, and embryonic and adult NEs on the other hand, imparts a spatial organization to NSC niches in the adult brain. In addition, in mouse, many RG populations become exhausted due to terminal differentiation, leading RGL NSCs to persist in even more restricted locations (Figure 4D). A typical example is that of the cortex: embryonic RG NPs terminate neurogenesis and terminally differentiate into astrocytes around E18 [60], and this contributes to isolating the SEZ and SGZ and non-contiguous neurogenic niches in the forebrain. Together, the spatial organization of adult NSC niches in the adult brain appears in part inherited from the lineage relation between NPs and NSCs, plus an exhaustion of some RGL pools between embryo and adult. This exhaustion varies between species, it is prominent in mouse and restricted in zebrafish. Of course, positive local cues (the so-called “niche”) also promote the persistence of adult neurogenic niches, such as in the SGZ and SEZ [61].
3.4. Take home message
NSCs are progeny cells of embryonic NPs. A comparative view of NP spatial organization in the embryo and adult sheds an ontological perspective on the spatial pattern of adult neurogenic niches, showing that it integrates a combination of events: spatial events that pattern the embryonic neural tube and impart progenitor properties that have long-term consequences on their progeny NSCs, and species-specific temporal events of local progenitor exhaustion, that further refine NSC niches to restricted domains in the adult brain.
4. The embryonic and adult neurogenesis cascade(s)
A primary molecular cascade controlling neurogenesis commitment is Notch signaling. It has been extensively studied and reviewed, in particular for its universal impact on embryonic neurogenesis [4, 54, 55]. The idea here is to mostly highlight its shared and divergent features between embryonic and adult neurogenesis, and to stress unknowns.
4.1. Neurogenesis drivers
In embryonic NPs, major neurogenesis drivers are basic Helix-Loop-Helix (bHLH) transcription factors of the Neurogenin (Neurog), Atonal (Ato), Achaete-Scute (Ascl) and NeuroD (specifically Neurod4) families [62]. These factors trigger the expression of a cascade of differentiation factors (such as Neurod1, 2 or 6) and effectors of neuronal functionality or identity (cytoskeletal elements, axonal differentiation, synaptic components, neurotransmitters etc) [63, 64, 65, 66]. Importantly, they are also the prime activators of expression of Notch ligand genes, thereby triggering Notch signaling [67]. Expression of proneural transcription factors is itself driven by neural Sox proteins such as Sox4 and Sox11, themselves signing neuronal engagement within permissive domains positive for Sox2 [68, 69]. Key parameters of this transcription factors cascade are its timing and tempo relative to other events such as cell cycle exit, progenitor delamination from the neural tube ventricle, and migration. The activity of proneural proteins is also modulated by phosphorylation, which can involve Cyclin-dependent kinases (Cdk) and hence be linked with cell cycle length. In particular, lower Cdk levels and cell cycle lengthening with age as embryogenesis progresses provide a window of opportunity for lower proneural protein phosphorylation levels and increased neurogenic efficiency [70]. In addition, proneural proteins are important counteractors of Notch signaling, discussed below.
4.2. Notch signaling principles, actors and effectors
Notch signaling is a cell–cell communication cascade, the outcome of which is to jointly regulate the state or fate of communicating cells. It is based on the interaction between a set of transmembrane receptors (Notch proteins [Notch1-4 in mammals, Notch1a, 1b, 2 and 3 in zebrafish]) and transmembrane ligands (Delta proteins [Dll1-4 in mammals, Dela-Deld in zebrafish] and Jagged proteins [Jag1-2 in mammals, Jag1a, 1b and 2 in zebrafish]). When expressed by contacting cells, the interaction between Notch and its ligand triggers the transmembrane cleavage of Notch to generate an intracellular fragment (NICD) that translocates to the nucleus, binds its interactor RBPj, and regulates transcription. This pathway has been extensively reviewed (for example: [71, 72, 73, 74]).
Multiple levels of regulation exist that involve post-translational modifications of Notch and its ligands, intracellular trafficking, degradations and recycling, and mechanical forces, that have been reviewed [75, 76, 77, 78]. An important feature here is that transcriptional Notch signaling is direct and non-amplified, i.e., the receptor itself serves as the transcriptional regulator, and one molecule of engaged receptor generates one molecule of NICD. Variations in the outcome are encoded by the Notch receptor used, the specific ligand–receptor pair that is engaged (see example below), the levels of available receptors and ligands, the specific dynamics of NICD interactions with the chromatin, and the cellular context (for example, the RBPj-bound loci open in a particular cell state, or the set of interactors available for Notch signaling targets). Another important feature of the pathway is that Notch signaling is an auto-consolidating system, as one output of signaling is to (directly or indirectly) enhance the expression of the receptor and ligand themselves.
General NICD/RBPj transcriptional targets are genes encoding transcription factors of the E(spl)/Hairy family (Hes and Hey in mammals, Her and Hey in zebrafish). These are basic helix-loop-helix (bHLH) transcriptional inhibitors that dimerize or pair with the ubiquitous bHLH protein E42 to downregulate target genes at E-boxes (CANNTG sequences) [63, 79, 80]. They themselves are among their main targets, thus terminating signaling. Some NICD/RBPj bound sites have been identified in mouse; in the context of embryonic NPs, they also include microRNA miR-9, itself an inhibitor of the neural progenitor state [81]; in adult NSCs, they include the progenitor gene Sox2 [82]. In the context of embryonic NPs and neurogenesis, Hes/Her themselves also inhibit expression of proneural genes. Because proneural proteins also positively control the expression of Notch ligands, this contributes to the consolidation of unidirectional Notch signaling. It is to note that, among all these interactions, very few direct transcriptional regulations have been formally demonstrated.
The main process involving trans interaction of Notch and a ligand is lateral inhibition [83, 84]. In embryonic NPs, it is classically initiated by the expression of a proneural factor, which upregulates expression of a Notch ligand. Signaling to a contacting Notch-expressing NP drives Hes/Her expression which in turn down-regulates expression of proneural and Notch ligand genes. This regulatory mechanism imparts and stabilizes distinct committed vs. progenitor fates to the Notch signaling vs. receiving cells, respectively. A contrasting trans-regulatory process, lateral induction, has also been described in embryonic NPs [85, 86]. There, Notch signaling in the receiving cell triggers expression of a Notch ligand, which propagates signaling to the neighboring cell, and so on from one cell to the next. This regulatory mechanism propagates a cell state across a cell population and has been described for example to define proneural domains of the embryonic inner ear [87]. Classically, lateral induction involves the Jagged ligand and an Hey1 effector, while lateral inhibition preferably relies on Delta ligands and Hes/Her. Finally, Notch and its ligands can also interact in cis, i.e., when expressed in the same cell [88]. Cis interactions decrease the number of receptors or ligands free to engage in trans interaction, thereby regulating the directionality and intensity of trans signaling.
4.3. Embryonic and adult neurogenesis: Notch signaling compared
Neurogenesis proper, i.e., the generation of a non-stem committed progenitor that further progresses towards the neuronal fate, is a transient event in the life of an adult NSC, which spends most of its time in quiescence (Figure 5A). Neurogenesis commitment can take place in association with NSC activation and division (in one or both daughter cells from a mother NSC), or independently of division. The latter case has been described in the zebrafish adult pallium where quiescent NSCs were observed to lose their astroglial character, delaminate from the ventricular zone and directly differentiate into neurons [10, 11, 89]. The mechanisms of direct differentiation remain unknown, and we will focus here on the generation of IPs from NSCs post-division.
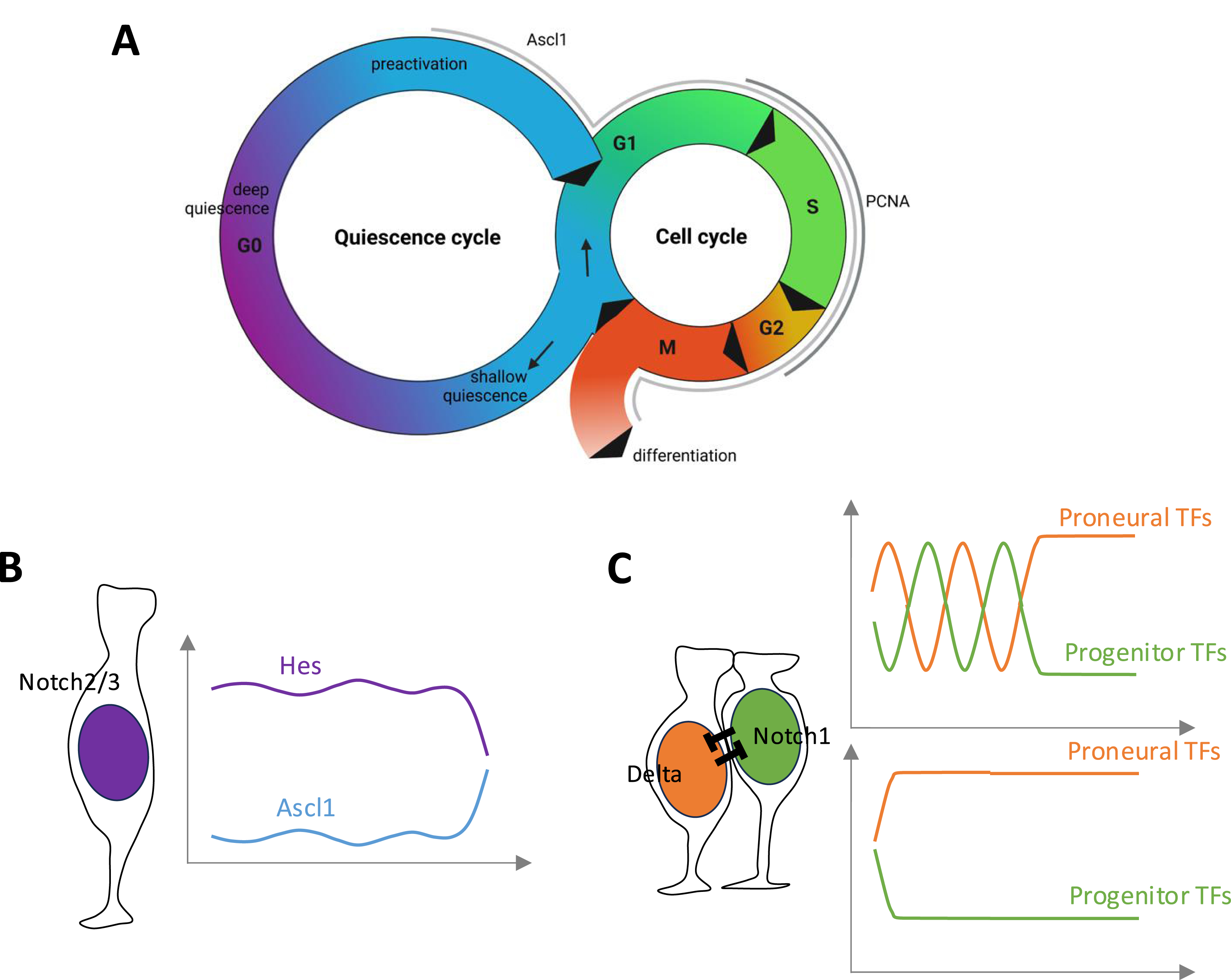
Cycling states, Notch signaling and fate choices during embryonic and adult neurogenesis (A). Quiescence (G0) and mitotic (G1-S-G2-M) cycles in neural progenitors. In the embryo, NPs are constantly cycling. In the adult brain, NSCs spend most of their time in quiescence and only infrequently activate to enter the mitotic cycle. (B) Adult NSC in quiescence, and levels of Hes and Ascl1 proteins (after [90]); quiescence is promoted by Notch2/3 signaling. (C) Fate choice in embryonic NPs or activated NSCs post-division. These choices involve Notch/Delta signaling where Notch1 is the primary Notch receptor. Signaling can be oscillating (top) or, as is probably the case for intralineage inhibition, directly oriented (bottom). Masquer
Cycling states, Notch signaling and fate choices during embryonic and adult neurogenesis (A). Quiescence (G0) and mitotic (G1-S-G2-M) cycles in neural progenitors. In the embryo, NPs are constantly cycling. In the adult brain, NSCs spend most of their time in ... Lire la suite
Embryonic RG NPs use Notch signaling when engaged in neurogenesis. The main proneural factors used in the forebrain are Neurog1 (ventrally) and Ascl1 (dorsally), and the main signaling players recruited are Notch1, DeltaA and Her4 in zebrafish (and Notch1, Dll1 and Hes1 in mouse). Other pathway members can be co-expressed, with partially redundant roles. For example, in the mouse embryo, it is necessary to knock-out the three genes Hes1, 3 and 5 to fully block neurogenesis [91]. Likewise, in the zebrafish embryo, single mutants have partial phenotypes (e.g., [92, 93]). In adult neurogenic niches, dividing NSCs express Notch1 and Her/Hes factors, and the conditional invalidation of Notch1 or RBPj in adult NSCs in the mouse SEZ and SGZ leads to NSC loss and differentiation [82, 94, 95]. Likewise, invalidation of notch1b in the zebrafish adult pallium leads to a loss of dividing NSCs and an increased production of neurons [96]. The key proneural factor expressed in the adult mouse SGZ and SEZ in dividing NSCs is Ascl1, and its direct requirement for the acquisition of the neuronal fate is discussed (see below). In the adult zebrafish telencephalon, where neurogenesis is found both in the subpallium and pallium, expression of neurog1 and ascl1a is found in subpallial and pallial NSCs [97, 98, 99, 100]. Thus, NPs and NSCs engaged in neurogenesis largely use similar neurogenesis pathway components, suggesting that the neurogenesis process involved during terminal division in NSCs mimics the one at play in embryonic NPs.
In addition, NPs and NSCs of the embryonic and adult brain rely on the molecular oscillatory properties of the Notch pathway to promote the generation of committed progenitors (Figure 5B,C) (recently reviewed in [101, 102]. Molecular oscillations are primarily driven by the self-inhibition of Her/Hes proteins, which inhibit their own transcription. In embryonic NPs, this leads to an oscillatory production of Her/Hes proteins with a period of ∼2–3 h. In turn, these oscillations drive offset oscillations of proneural factors. Current evidence in the mouse and zebrafish embryos suggests that these oscillations of antagonistic fate drivers maintain NP in a plastic state poised for a fate decision, whether to remain an NP by stabilizing Notch signaling, or to commit to neurogenesis by stabilizing proneural expression. Several mechanisms have been postulated to underlie this final choice. Among them, microRNA-9 (miR-9), itself in a negative transcriptional feedback loop with Her/Hes factors but whose stability permits accumulation, may serve as a dose-dependent “clock” mechanism that will tilt the equilibrium towards neuronal commitment when above a threshold [103]. Another parameter is cell cycle length, which increases with time thereby allowing changes in the activity of proneural factors and/or cell cycle exit decisions (see below).
Most interestingly, alternating oscillations of Hes1 and Ascl1 proteins have also been observed (by means of Hes1-Venus and Ascl1-Venus fusion proteins using the endogenous Hes1 and Ascl1 loci) in dividing adult NSCs of the mouse SGZ [90, 104, 105]. The experimental induction of Ascl1 oscillations drives NSCs to enter the cell cycle and permits neurogenesis, and Ascl1 itself is absolutely required for NSC proliferation [106, 107]. Compared to embryonic NPs, it is less clear in adult NSCs whether Ascl1 itself encodes the neuronal fate, given that it appears very transiently expressed in IPs [31, 108], and that its overexpression rather induces oligodendrogenesis [109, 110]. It also remains unclear how oscillations drive proliferation, and how they are initiated and stopped. Regarding proliferation, the co-expression of Ascl1 and NICD may render the regulatory elements of proliferation genes accessible to Ascl1, as observed in embryos [111]. Regarding the initiation of oscillations, it was noted that the Ascl1 mRNA is in fact expressed at low levels in most NSCs but is not productive due to post-translational destabilization of the Ascl1 protein [107]. This block is relieved pre-division, and this release may be sufficient to initiate a default oscillatory behavior. Finally, it is interesting to note that Notch1 seems to be the primary Notch receptor involved in progenitor maintenance during the activated phase of adult NSCs, both in mouse and zebrafish [96, 112]. Together, these observations highlight similarities between embryonic NPs and the activated and neurogenic state of adult NSCs.
4.4. Cell cycle length and cell cycle exit
In embryonic NPs, neuronal commitment and cell cycle exit are not necessarily concomitant but are coupled in several ways. First, the progressive lengthening of the cell cycle and decreased Cdk activity during embryogenesis permit the accumulation of non-phosphorylated Sox2 (less active) [113] and proneural proteins (more active) [70], thereby promoting the switch from the progenitor to the differentiated state. Cell cycle lengthening itself depends on a Cdk-independent function of mitosis phosphatases such as Cdc25b [114]. In turn, proneural proteins, in addition to commitment, can control the expression of cell cycle components. This is the case of Ascl1 in mouse NPs in culture where, depending on the presence or absence of Notch signaling in its expressing cell, it can activate the expression of cell cycle-promoting genes (e.g., CyclinD1) or of genes inhibiting cell proliferation [111]. Second, Cyclins can also participate in transcription complexes and/or directly bind DNA to control the expression or activity of commitment proteins. For example, CyclinD1 can associate with (and inhibit) NeuroD [115].
In adult NSCs, direct neuronal differentiation events from quiescent NSCs have been observed [10, 11, 89]. The mechanisms of this fate decision, which temporally uncouples commitment from cell cycle exit, remain to be identified. For IP-generating NSC divisions, daughter cell fate choice is generally not made between cycling and commitment, but rather between quiescence (G0) and commitment. We note, in the mouse SEZ, the important role of two Cyclin-dependent kinase inhibitors, p21 and p27. p21Cip, in addition to its cell cycle-related function, inhibits expression of BMP2 and Sox2, preventing the exhaustion of SEZ NSCs otherwise taking place through excessive expansion or astrocytic differentiation, respectively [116, 117]. These inhibitions involve Cdk-independent functions of p21, including direct binding to the Sox2 enhancer. In epithelial cells in culture, endogenous p21 levels, which accumulate in G2, bias the fate of daughter cells towards G0 vs. G1 [118, 119]. So far however, this was not reported in adult NSCs. In the SGZ, p27 is necessary for NSC quiescence and for cell cycle exit of IPs through its regulation of the cell cycle [120], but it also directly binds to (and inhibits) the Sox2 promoter to promote the transition of IPs to differentiation [121].
In the context of the relation between cell cycle components and commitment, one key issue in both embryonic NPs and adult NSCs is to understand how a different choice between commitment and progenitor NP/NSC maintenance can be imparted in sister cells, in the case of asymmetric divisions. Rather than driving self-renewal or commitment, the factors above may open susceptibility states prone to fate choices, which are then made in daughter cells following intralineage or environmental cues (see below).
4.5. Take home message
Notch signaling, Hairy/E(Spl) factors and proneural proteins are major determinants of the choice between progenitor maintenance and neurogenesis progression in both embryonic NPs and active adult NSCs. These pathways are intertwined with cell cycle regulatory components to coordinate cell cycle exit and lineage progression. The quiescence state of adult NSCs complexifies this regulation by introducing a triple choice post-division: to re-divide, to re-enter quiescence, or to commit.
5. Division modes or how to balance progenitor maintenance and recruitment
5.1. Division modes in embryonic NPs and adult NSCs
We will consider here divisions relative to the generation of IPs (or neurons when there are no intermediates). Hence, NSC/NSC and NP/NP divisions will be called symmetric amplifying, NSC/IP or NP/IP asymmetric, and IP/IP symmetric differentiative.
In the embryo, NP divisions of the three types above were reported in vivo (Figure 6A). Typically, NE progenitors divide in a symmetric amplifying manner, enlarging the NP pool. In contrast, RG NPs divide asymmetrically to generate one RG and one IP or neuron [60, 122]. In the zebrafish embryo, this process has been filmed in the telencephalon and hindbrain [123, 124]. In addition, at early stages, at least in zebrafish, some NPs divide following a symmetric differentiative mode, generating two neurons used to build the first larval neuronal scaffold [125]. The corresponding NPs are therefore lost in the process.
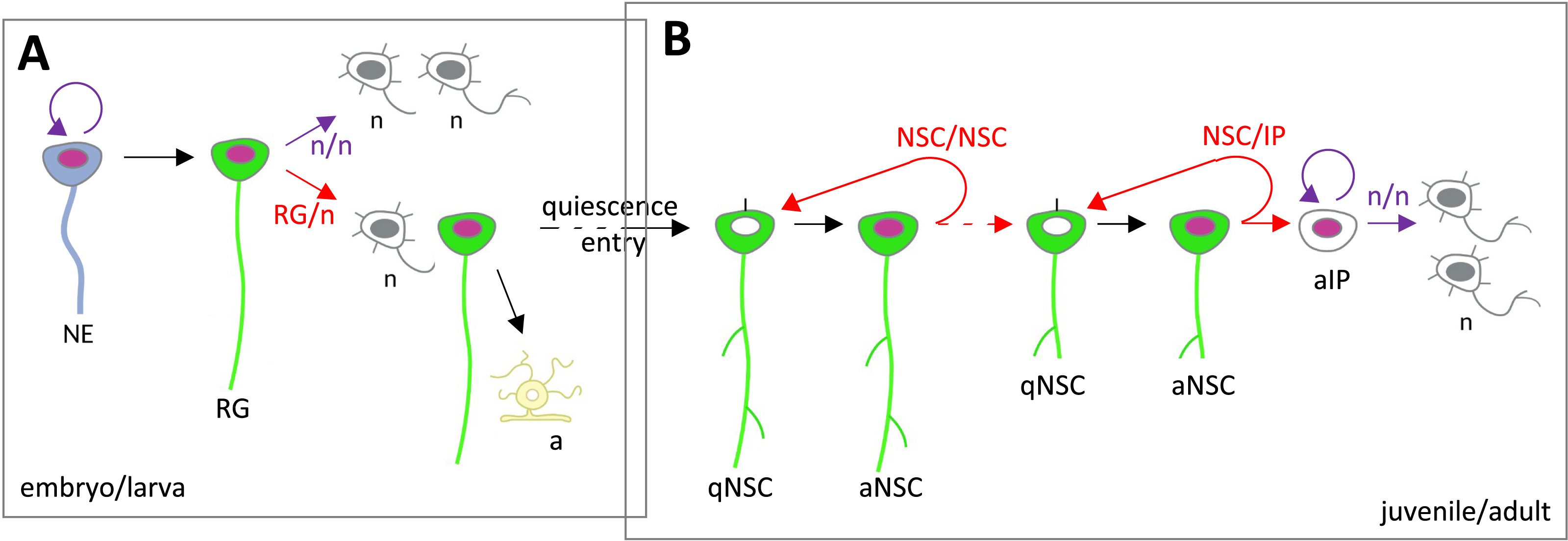
Lineage progression and division modes during embryonic and adult neurogenesis. (A) In the early embryo, NEs divide symmetrically, while the predominant division mode of RGs is asymmetric self-renewing to generate one RG and an IP or a neuron daughter. At late embryogenesis in the mouse, most RGs differentiate into astrocytes, and some enter quiescence to give rise to adult NSCs. In zebrafish, it is believed that most neurogenic RGs transit to the NSC state. (B) In the adult, NSCs undergo NSC/NSC-generating divisions. NSCs also progressively commit to give rise to NSCs that will generate IPs and, in turn, neurons. In the zebrafish adult pallium, an upstream self-renewing and asymmetric NSC/NSC division has been identified [126], likely responsible for the homeostatic maintenance of the NSC population. Symmetric divisions are drawn in purple, asymmetric divisions in red. Abbreviations: a, astrocyte; IP, intermediate progenitor; n, neuron; NE, neuroepithelial progenitor; aNSC, activated neural stem cell; qNSC, quiescent neural stem cell; RG, radial glia. Masquer
Lineage progression and division modes during embryonic and adult neurogenesis. (A) In the early embryo, NEs divide symmetrically, while the predominant division mode of RGs is asymmetric self-renewing to generate one RG and an IP or a neuron daughter. At ... Lire la suite
Likewise, the three division modes take place in adult NSCs, in both the mouse and zebrafish forebrain (Figure 6B) (reviewed in [27]). They have been directly observed using intravital imaging in the adult mouse SGZ and zebrafish pallium [10, 11, 89, 127, 128, 126]. Modeling clonal dynamics in the zebrafish adult pallium predicts that NSCs choose stochastically between these division modes, in specific proportions [89]. Intravital imaging tracking validated these predictions, both regarding proportions and the apparently random order in which symmetric amplifying and asymmetric divisions occurred in tracks with multiple divisions [126]. In contrast, in the adult mouse SGZ, intravital imaging revealed non-stochastic choices, e.g., the fact that symmetric amplifying divisions never followed asymmetric divisions [128].
5.2. Population vs. intralineage fate regulations, and implications
Daughter cell fate choices can be imparted by the mother cell (e.g., through the partitioning of fate determinants), organized between daughter cells post-division through intralineage interactions, or biased in a non-cell autonomous manner by the surrounding cells or niche. To distinguish between these regulations, it is necessary to trace individual NPs/NSCs in situ and modify their signal sending or receiving properties. A major cell–cell signaling pathway involved is Notch.
In the embryonic neural tube, the division of individual NE progenitors has not been tracked; however, the fact that Notch signaling—at least in the mouse neural tube—becomes active at a relatively late stage, after the initial NE amplification phase, strongly suggests that a lateral inhibition process takes place between adjacent NEs irrespective of the closeness of their relationship (Figure 5C, top, and Figure 6A) [129]. At the RG stage, blocking DeltaA expression in individual RG of the telencephalon leads to neuronal differentiation of all cells in each clone after just a few divisions, while control clones maintain a RG identity [123]. This approach prevents Notch signaling from occurring between clonally related cells but not with their non-clonally related neighbors (which express Delta, while cells of the clone express Notch), allowing to conclude that division asymmetry results from Notch signaling preferentially occurring between sister cells. This has been called “intralineage regulation” (Figure 5C, bottom, and Figure 6A). It is likely also taking place in the embryonic hindbrain. Further functional assays demonstrated that it requires the asymmetric distribution of the Delta ubiquitin ligase Mindbomb (Mib), itself permitted by the asymmetric distribution of Par3 at division [123], which follows DeltaD-carrying recycling endosomes, in a complex that necessitates the centrosomal and endosomal protein PCM1 [130, 131]. In zebrafish (but not in mouse), Mib and Par3 are inherited by the future neuron. In a parallel set of studies, asymmetric distribution of DeltaD between sister cells was observed during RG division in the embryonic zebrafish hindbrain. DeltaD is transported by Sara endosomes, which are themselves partitioned unequally at division [132]. What prevents cells from interacting with their non-lineage related neighbors is not clear. It could be that the mechanisms involved in partitioning Notch–Delta signaling components between sisters at division dominate, in strength or speed, the establishment of other interactions. Asymmetric distribution of Dll1 protein was also observed (on static preparations using immunostaining) in dividing RGs of the mouse embryonic cortex.
The mechanisms that account for terminal division asymmetry (NSC/IP or NSC/neuron) in the adult brain have not been studied in vivo to date. In cell pair assays in vitro, asymmetric Notch signaling levels can be observed between mouse SEZ NSC daughters. This study identified that Pigment epithelium-derived factor (PEDF), possibly released by endothelial and ependymal cells of the niche, induces NFkB activation, which itself leads to release of inhibition at Hes1 and EGFR promoters, permitting Notch signaling and EGFR expression [133]. This mechanism, where differential proximity to PEDF might introduce asymmetries between sisters, is superimposed on the effect of the Dyrk phosphatase, which affects the stability of EGFR and decreases self-renewal [134]. In a possible link with this, asymmetric distribution of the Dll1 protein was observed in GFAP+ NSCs of the mouse SEZ [135]. In Dll1+ dividing mother cells (at pre-cytokinesis stages), an asymmetric distribution of Dll1 was seen in a majority of cases.
5.3. Take home message
The control of the NSC/NP division mode (in relation with the generation, or not, of an IP or neuron) relies on a combination of intrinsic determinants, privileged sister-sister interactions or more general local cues. Notch signaling is an important regulatory pathway in all scenarios. Its implication in the control of division modes in adult NSCs in vivo remains to be formally demonstrated.
6. Lineage progression, conserved and changing progenitor properties over time
6.1. Commitment status along lineage progression during adult neurogenesis
Our recent work based on intravital imaging to track NSC behavior in the adult zebrafish pallium points to another level of complexity in the control of the NSC division mode. Indeed, symmetric NSC/NSC divisions were observed to generate differently fated NSC daughters (Figure 6B). Specifically, NSCs negative for the expression of the Notch ligand DeltaA (deltaAneg) divide to generate one deltaAneg daughter NSC seemingly identical to its mother, and one daughter that switches on expression of deltaA (deltaApos) and will further divide at higher frequency to finally generate IPs [126]. The divisions of deltaAneg NSCs are therefore asymmetric in terms of NSC fate, splitting daughter NSCs between self-renewal and lineage progression. The mechanisms controlling this asymmetric division mode remain unknown. Furthermore, the division of deltaApos NSCs was seen to generate daughters with progressively increasing levels of deltaA expression, possibly indicating progressive neurogenesis commitment during divisions. These levels could differ between daughters, which may also signify different commitment levels. Although our understanding remains very limited, and heavily relies on our limited readouts of cell state or identity, these observations together raise the fundamental question of whether bona fide symmetric NSC divisions exist at all during adult neurogenesis.
6.2. The notion of self-renewal
The notion of self-renewal is associated with the definition of a NSC, hence largely with the adult context. Probing for it is however limited by our experimental readouts. Whether molecular, cellular or functional, these readouts are only very partial accounts of a cell’s state. It is within the frame of these technical limitations that deltaAneg NSCs of the adult zebrafish pallium are currently considered to self-renew (Figure 6B) [126], but more precise analyses of these cells in the long-term would be needed. In this context, ageing is an interesting aspect to study. Zebrafish pallial NSCs are described to activate less frequently in aged animals [136, 137], but NSC state under precise ageing conditions remains largely unexplored at the molecular level. Changes in activation frequency and potential have also been reported in aged NSCs in mouse [138, 139, 140, 141]. However, self-renewal proper has not been studied in either species.
Embryonic NPs, except for those that will ultimately give rise to adult NSCs, are bound to exhaust after the generation of neurons. This can occur after a fixed number of asymmetric divisions, as described in the mouse embryonic cortex where RG undergo on average 8 divisions to generate 8–9 neurons [142]. It is difficult to imagine that daughter RGs arising at each division are strictly identical to their mother until the end, as some mechanism must register each round of cell division, and in fact, the duration of the cell cycle increases over time. In the mouse embryonic cortex and retina, the transcription factors expressed by NPs also change over time and divisions [143, 144], akin to the transcription factor series deployed to generate different neuronal subtypes during nerve cord or optic lobe neurogenesis during fly development [145, 146].
6.3. Quiescence and quiescence instatement
A striking feature of adult NSCs, compared to embryonic NPs, is their prolonged quiescence (Figure 5A) [147]. Thus, to initiate the neurogenesis process, adult NSCs first need to exit the quiescence state, and their quiescence/activation balance is an additional level of control compared to embryonic neurogenesis.
Adult NSCs are stalled in G0, in which they spend most of their time. In the adult mouse SGZ for example, tracing RG-like NSCs shows a mean duration between two cell cycles (for dividing NSCs) varying between 1 and 90 days [128], and this amounts to a doubling time of 124 days for the highly quiescent NSC subpopulation in the zebrafish pallium [126]. Several pathways were identified to control the quiescence/activation balance, among which Notch2/3 signaling, BMP signaling, and Id factors promote quiescence [96, 148, 149, 150, 151, 152, 153], while mTORC1 and EGF signaling and the transcription factor Ascl1 promote activation [154, 155, 156, 157, 107]. In detail, the Notch ligand DeltaA is expressed by IPs in the adult zebrafish pallial niche, and IPs signal via Notch to prevent activation in contacting NSCs [158, 159]. As mentioned above, deltaA is also expressed in the subpopulation of NSCs engaged in neurogenesis, and these cells also maintain their quiescence via Notch signaling, possibly tempered by a cis-regulation by DeltaA [126]. In zebrafish, BMP4, produced by pallial neurons, acts in concert with Notch, possibly via the induction of expression of Id1 [150]. Id4 plays a similar role in mouse [149]. Notch signaling and Ascl1 are particularly interesting to consider in the context of this review, as they have been extensively studied in embryonic progenitors and permit a direct comparison. While Notch2/3 maintain quiescence, Notch1 is expressed in activated NSCs and maintains their progenitor potential, as the selective abrogation of Notch1 function triggers neuronal differentiation [94]. In parallel, low levels of Ascl1 transcription are found in quiescent NSCs, while higher and oscillatory expression of Ascl1 is sufficient to trigger activation [90, 106, 128]. Because the involvement of Notch1 and Ascl1 oscillations are reminiscent of neurogenesis control in embryonic NPs, these observations suggest that, at adult stage, activated NSCs engaged in neurogenesis recapitulate an embryonic process, and that new regulators have been superimposed on this initial embryonic regulatory cascade to encode the quiescence state in adult NSCs.
Quiescence duration is highly variable between NSCs, with overall much longer doubling times for NSCs of the deltaAneg self-renewing pool (123 days) than for the deltaApos neurogenic pool (28 days) in the adult zebrafish pallium [126]. In addition, NSCs activate more or less rapidly upon Notch blockade, suggesting the existence of different quiescence depths [96]. Thus, compared to embryonic NPs, adult NSCs not only display a quiescence phase but different quiescence durations and/or depths. The regulation of this heterogeneity is only beginning to be unraveled. It is a complex task given that, in vivo, quiescence parameters such as duration are linked with NSC progression along the lineage, hence these aspects are difficult to disentangle.
Finally, it remains important to understand how quiescence is instated in NPs that transit to NSCs during life. Classically, quiescence is measured at the population level as the proportion of NPs found in the non-activated state (PCNAneg or MCM2/5neg) at any given time within a population. With such measurements, quiescence became detectable between larval day 5 and 8 in the zebrafish pallium (50% PCNAneg RGs) to progressively increase during the juvenile period to finally reach adult levels (typically 5% of PCNAneg or MCM2/5neg NSCs) [160]. Individual NPs/NSCs have not been tracked, however, and they divide asynchronously. Thus, whether the increased proportion of PCNAneg or MCM2/5neg NSCs with age reflects the progressive increase of quiescence duration in each cell over time, or the asynchronous entry of individual cells into an adult-like duration of quiescence, cannot be concluded at this point. If, as suggested above, quiescence is the result of the recruitment over time of an additional molecular pathway (such as Notch2/3) by NPs, another open question is what triggers the expression of this pathway.
6.4. Take home message
Lineage progression involves intrinsic changes in NP/NSC properties, affecting their commitment level, the identity of the neurons they generate, or, in the adult brain, the depth or duration of quiescence. These changes occur concomitantly and are experimentally difficult to disentangle. Another question that remains open is their relationship with cell division. Steps in lineage progression are often observed to take place concomitantly with cell division, e.g., with at least one daughter appearing to differ from its mother. However, whether lineage progression also occurs between cell divisions remains to be studied.
7. Environmental/systemic/large-scale populational regulation
NPs/NSCs and the neurogenesis process are controlled by cell-intrinsic molecular and cellular events, as well as local cell–cell interactions, as discussed until now. On a larger spatial scale, NPs/NSCs are also embedded in a niche, which is a key regulator of their fate.
An important component contacting NPs and NSCs in both embryos and adults is the cerebrospinal fluid, largely produced by the choroid plexuses and containing a spatially heterogeneous mix of bioactive molecules (notably hormones and growth factors), peptides and neurotransmitters that are either filtered from the blood or secreted by the choroid plexuses themselves, the supracommissural organ, tanycytes, or neurons. NPs/NSCs themselves release factors or vesicles into the CSF [161, 162, 163]. Many of these factors, whether brain-borne or blood-borne, in turn impact the proliferation or commitment of NPs/NSCs or downstream steps of the neurogenesis process. Steroid hormones, thyroid hormone, insulin, cortisol, growth hormone, FGF/EGF etc, have been the focus of intense study in this context. Hormones vary between the embryonic and adult contexts, also because in embryos some are mother-derived (released during pregnancy in mammals, or deposited in the oocytes in fish for example). They will not be discussed here as they do not affect the principles of neuron generation as such, but they are clear modulators of neurogenesis at the systemic level.
Additional to this external, systemic niche, our recent work in the zebrafish adult pallium led us to postulate the existence of a medium-scale “intrinsic niche” created, via spatiotemporal feedbacks, by NSCs and IPs [164]. By restricting NSC activation in the vicinity of lineage intermediates (IPs), a specific number of activated NSCs is permitted at any given time, and is positioned in physical space within the NSC population. Spatial modeling suggests that this regulation is important to avoid spatial drifts in neuronal production in the long-term [159]. At this point, the demonstrated interactions are local, and whether and how they propagate across the NSC population is expected to depend on the relation between the frequency and duration of NSC states competent for activation and the half-life of IPs. Long sub-apical protrusions have also been observed in deltaApos cells that could signal at a distance [126]. An interesting aspect of these findings is that spatial signaling is temporally linked with lineage progression. Thus, in the asynchronous adult NSC population where IPs are also short-lived (and leave the germinal population upon differentiation), this allows a self-perpetuating system to emerge where lineage termination allows lineage initiation from another NSC. The spatio-temporal coordination of NSC fate decisions, other than their activation, remains to be tested, as well as the relevance of other signaling pathways, whether relying on cell–cell interactions, reaction–diffusion processes, or mechanical information. Finally, in addition to this spatio-temporal regulation at the individual cell level, it is interesting to wonder whether the intrinsic niche also includes spatio-temporal feedbacks at a clonal level. Such regulations akin to competition processes have been described in non-follicular epidermal SCs of the mouse adult skin [165].
In the embryonic central nervous system, the occurrence of cross-interactions between NPs to control the position of neuron generation in physical space is a well-known property of lateral inhibition. In contrast to the adult pallial NSC pools, obviously, it affects neurogenesis commitment rather than quiescence exit. In addition, Notch-Delta signaling via horizontal protrusions exhibited by NPs were recently described to space out neuronal differentiation in the zebrafish embryonic hindbrain, showing that lateral inhibition can also generate longer-distance patterns [166].
7.1. Take home message
In addition to systemic or local signals from non-NPs/NSCs, the fact that NPs/NSCs are often found in groups allows inter-progenitor interactions. This “intrinsic niche” is responsible for the organization of neurogenesis progression in physical space over time.
8. Conclusion
The core principles and molecular regulators of neurogenesis are shared between the embryonic and adult central nervous systems, with some adaptations in spatial organization and speed. The latter is much slower in the adult compared to the embryonic brain, due to a number of factors including the existence of an NSC quiescence phase and a lengthened progenitor commitment phase. Overall, the neurogenesis process is better understood, because experimentally more easily tractable, in the embryonic than in the adult brain. But the next step and recurrent remaining issue is to understand transitions: how are cell identities gradually changed, how is cycling behavior modified (to introduce quiescence), how are lineages prolonged (to introduce transient progenitor states) in the transition from embryo and adult? Are these transitions abrupt or gradual, what are their regulators, and how is a new equilibrium reached that will characterize the adult stage? Future work along these lines will be highly informative for understanding how dynamic pattern and tissue generation concert over time to result in tissue homeostasis.
Abbreviations
| bHLH | Basic helix-loop-helix domain |
| BLBP | Brain Lipid-Binding Protein |
| BMP | Bone morphogenetic protein |
| Cdk | Cyclin-dependent kinase |
| CSF | Cerebrospinal fluid |
| Dll1 | Delta-like 1 |
| EGF | Epidermal growth factor |
| EGFR | Epidermal growth factor receptor |
| FGF | Fibroblast growth factor |
| GFAP | Glial fibrillary acidic protein |
| GS | Glutamine synthase |
| Hes/Her | Hairy/Enhancer-or-Split transcription factor |
| INM | Interkinetic nuclear migration |
| IP | Intermediate neuronal progenitor |
| MHB | Midbrain–hindbrain boundary |
| NE | Neuroepithelial cell |
| NICD | Notch intracellular domain |
| NP | Neural progenitor |
| NSC | Neural stem cell |
| PEDF | Pigment epithelium-derived factor |
| RG | Radial glia |
| SEZ | Sub-ependymal zone of the lateral ventricle |
| SGZ | Sub-granular zone of the dentate gyrus of the hippocampus |
| ZO1 | Zona Occludens 1 |
Methods
Images in Figures 2, 4, 5 and 6 were generated using BioRender (with a license from Institut Pasteur).
Declaration of interests
The authors do not work for, advise, own shares in, or receive funds from any organization that could benefit from this article, and have declared no affiliations other than their research organizations.
Author contributions
Conceptualization and writing—original draft—: LB-C; review & editing: MC, LB-C; illustrations: MC.
Funding
Work in the LB-C laboratory was funded by the ANR (Labex Revive), La Ligue Nationale Contre le Cancer (LNCC EL2019 BALLY-CUIF), the Fondation pour la Recherche Médicale (EQU202203014636), CNRS, INSERM, Institut Pasteur and the European Research Council (ERC) (SyG 101071786 - PEPS). MC was a recipient of a 3-year PhD student fellowship from ED Complexité du Vivant, Sorbonne Université, Paris, complemented for one year by LabEx Revive.
Acknowledgments
We thank the ZEN team for generating some of the important scientific data mentioned in this review, and for regular discussions.





 CC-BY 4.0
CC-BY 4.0
Vous devez vous connecter pour continuer.
S'authentifier