1 Introduction
In birds, the circadian system seems to be composed of three main elements: a sensitive input, oscillators and a behavioural or physiological rhythmic output. It is thought that the pacemakers are coupled together via different neural and hormonal pathways [1]. Under photoperiodic conditions, light information is received by the sensitive pathways (input) and synchronizes the circadian oscillators. These sensitive pathways are multiple, complex and distinct from those involved in the processing of visual information [2].
In many Vertebrates, photoreceptors are present in the retina, the pineal gland and in the hypothalamus and have been considered to be involved in periodic phenomena. In birds, transmission of light information by extra-ocular photoreceptors appears essential for seasonal reproduction and regulation of circadian physiology [3–6]. Local illumination showed that the pineal [7] and the medio-basal hypothalamus [8] in quail and the hypothalamus in duck [9] are photosensitive for photoperiodic gonadal responses. Moreover, in non-mammalian species, light can directly control pineal function, including the synthesis and release of melatonin [10,11].
In the Japanese quail, the retinal photoreceptors play a major role in the regulation of the circadian rhythm of locomotor activity under photoperiodic conditions [12]. But, bilateral enucleation of Japanese quail did not abolish the circadian rhythm of body temperature in photoperiodic conditions [13]. Thus, it appears that the photoreceptive structures are involved in the rhythm synchronization with light condition changes according to the biological rhythm studied.
The laying behaviour of Japanese quail shows a temporal organization synchronized with the photoperiodic conditions [14]. Recent experiments showed that ovipositions occur during a species-specific laying window situated, in LD 14:10, between 7 to 14 h after the light was switched on [15]. Inside this laying window, some females lay each day at the same hour. Their stable rhythmic laying profile enables research on the possible sensitive input.
The aim of this study was to test the effect of a light cycle on the laying rhythm in quail by stimulating only a small part of the skull. For that, we elaborated a device containing a diode emitting a unidirectional light beam and stuck it on the top of the cranium, the birds being maintained in standardized conditions.
2 Materials and methods
2.1 Animals and housing
We used ten mature domestic Japanese quails (Coturnix c. japonica) females. Previous experiments showed that out of 102 females reared in LD 14:10, 80% showed a ‘stable’ laying profile, characterized by a same laying time each day and therefore an oviposition interval (time between two successive eggs) close to 24 h. The remaining 20% of birds show a ‘delayed’ laying profile, characterized by ovipositions occurring progressively later each day and then, an oviposition interval greater than 24 h [15].
The ten birds were therefore selected for presenting a ‘stable’ laying profile with laying times that were relatively identical (from 9 to 11 hours after the light went on, depending on the female). The quail were placed in a battery with individual cages (25×20×15 cm). The battery was in a soundproof room with neon lighting. The temperature was constantly maintained at 20±1 °C. Food and water were supplied ad libitum.
2.2 Experimental protocol
At first (phase 1), the quail were maintained during 15 days under photoperiodic conditions LD 14:10 (see Fig. 1; protocol on the right part). During the photophase, the light intensity emitted by the neon was 550 lux. At the end of this phase, the devices were put on the quails' cranium. After 48 h of dim green light (5 lux), the birds were maintained in constant darkness (DD) until the end of the experiment. The transitional phase with dim green light let us to control that quails normally behaved and especially fed without bright lighting. In DD, for certain females, the devices were switched on during 17 days (phase 2), for 14 h a day, simulating a photoperiod of 14 h of light and 10 h of darkness. During the ten following days (phase 3), the devices were switched off continuously. During this phase 3, the birds were visited and handled twice a day, at the times corresponding to the switching on and the switching off of the devices during phase 2.
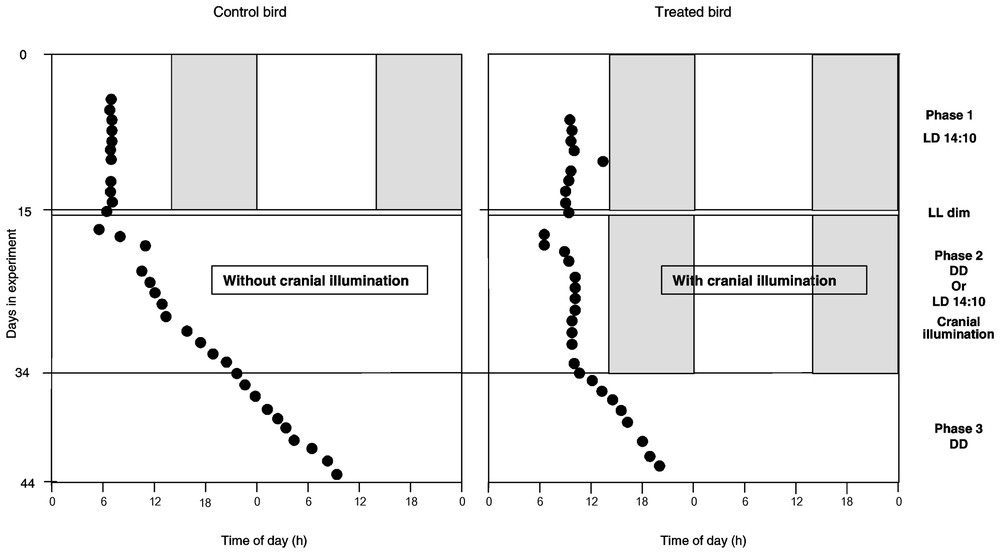
Evolution of laying time of successive eggs (dark circles), defined in accordance with ‘light-on’ time, during the three phases of the experiment in a control bird (left) and in a treated bird (right). Details of protocol schedule on the right.
2.3 Device of unidirectional light stimulation
We elaborated a device composed of two elements (Fig. 2a). The first part was stuck on the cranium of the bird (Fig. 2b). It was composed of a diode emitting a white light (LED Everlight EL204 VWC) and a reflector in order to focus the light emission on the skull. The external side of the reflector was painted black and covered with a thick fabric in order to prevent the light from diffusing out. The light intensity of the LED was adjusted (140 lux) according to the light intensity received by the bird in the cage from the room neon placed in front of the cages during phase 1. The second part was stuck on the back of the bird (Fig. 2b). It was composed of a lithium battery of 3 V (Varta CR2025) placed on a plastic support. The LED was supplied by the battery via a resistor of 3.3 k. The current through the LED was 0.17 mA. On the support, there was a plastic part composed of a socket and a plastic hole. The two parts of the device were connected with an electric wire using a plug. We plugged it either into the socket, in order to switch on the LED, or into the plastic hole, in order to switch off the LED. Thus, the electric wire was always joined to the device, in order to avoid disrupting the birds and to prevent the wire from being damaged by bird movement.
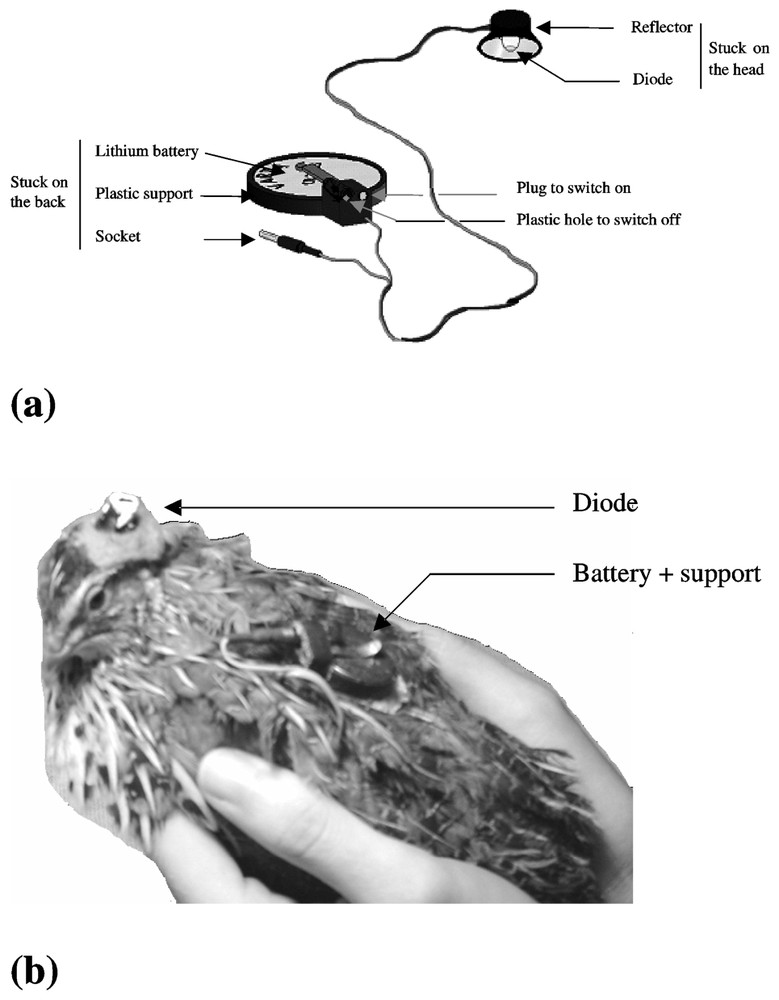
Device of unidirectional light stimulation alone (a) and placed on the animal (b).
We removed some feathers from the cranium and from the back of the bird to improve the adhesion of the device to the skin. We stuck it with ‘super glue 3’. After sticking the device, we checked that the birds did not express discomfort behaviour.
At first, all ten quail were equipped with the device, considering that each bird during phase 3 could be its own control compared to phase 2. But as five birds moved their devices a little during the first days of phase 2 when the LED was switched on, a low light could diffuse. In order to avoid possible light stimulation of ocular photoreceptors, we decided not to use these devices and the birds in question were considered as a control group (N=5) during the whole experiment, compared to the five stimulated females (treated group).
2.4 Laying behaviour
The laying time was automatically recorded by a system of cameras linked to a microcomputer (Commodore, Amiga). Ten infrared cameras, one in front of each quail, sent a picture every 4 s to the computer. Software, developed in our laboratory, enables screen visualization of a mosaic of ten pictures (160×128 pixels), allowing simultaneous observation of the ten birds. A record of the screen was taken every minute. The pictures were studied once a day.
2.5 Data analysis
Temporal laying behaviour can be characterized by two parameters: laying time and oviposition interval. By recording with cameras, the laying time can be estimated to about one minute. Nevertheless, sometimes the egg could not be observed because the bird was holding it under its belly. This situation could last for a maximum of 10 min. In this case, the laying time was defined as the mean time between the time of the last picture without an egg and the time of the first picture with an egg. So, laying time was evaluated with an error of ±5 min (=0.08 h rounded up to 0.1 h). Oviposition interval was estimated as the time separating the laying of two consecutive eggs. It corresponds to the period of the laying rhythm. In constant conditions, oviposition interval is the one parameter used for characterizing laying behaviour.
The data were analysed using non-parametric tests for independent (Mann–Whitney test) and dependent (Wilcoxon test) data [16].
3 Results
The mean number of laid eggs per female during the three phases of the experiment is described, for each group, in Table 1.
3.1 Laying interval
Examples of laying profile for a female of each group are shown in Fig. 1.
During phase 1 in LD 14:10, as expected, all the females expressed an oviposition interval of approximately 24 h (24.0±0.1 h, N=10) (Fig. 3). No significant difference was noticed between the oviposition intervals of the females of the two groups (24.0±0.1 h, N=5, in each case) under photoperiodic conditions (Mann–Whitney, p=0.17, N=10).
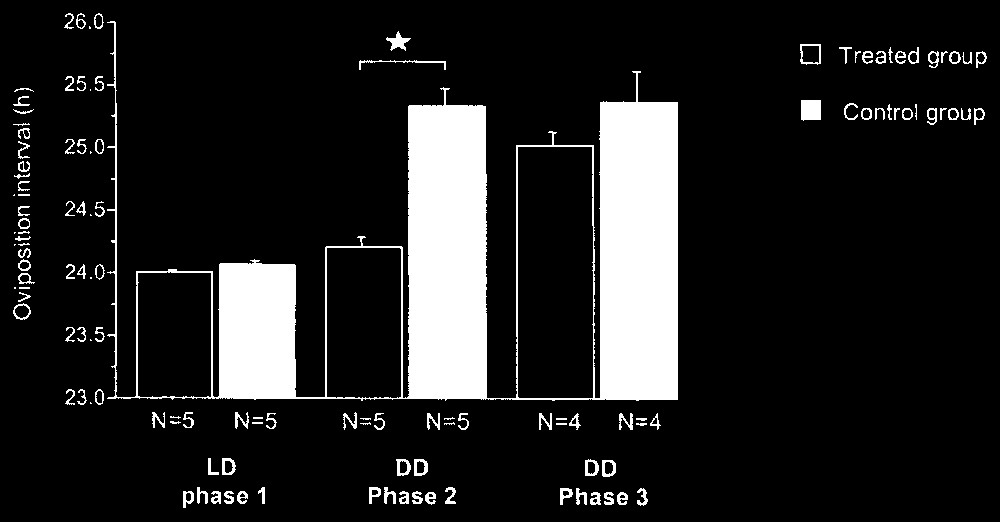
Oviposition mean interval (+ES) for females of treated and control groups during the three experimental phases. : Mann–Withney, p<0.05.
During phase 2, in DD, the females of the two groups (without or with rhythmic activity of the LED) presented significantly different oviposition intervals (Mann–Whitney, p=0.009, N=10) (Fig. 3). All the females of the control group showed a significant increase of oviposition interval between phases 1 and 2 (Wilcoxon, p<0.05) with a mean value of 25.3±0.1 h (N=5) and so expressed their free-running periods. The females of the treated group maintained the same oviposition intervals between phases 1 and 2 (Wilcoxon, p>0.05) with a mean value of 24.2±0.1 h (N=5).
During phase 3, in DD (without rhythmic activity of the LED), one female from each group stopped laying. The birds of the two groups showed similar periods of laying rhythm (Mann–Whitney, p=0.30, N=8) (Fig. 3). The females of the control group did not present a significant difference for oviposition interval between phases 2 and 3 (Wilcoxon, p>0.05) with a mean identical value of 25.3±0.2 h (N=4). The oviposition interval increased between the two phases for all the birds in the treated group (Wilcoxon, p<0.05) with a mean value of 25.0±0.1 h during phase 3 (N=4). These laying intervals corresponded to those of free running birds in DD, i.e. about 25 h [17].
Thus, during phase 2, the females of the treated group synchronized their laying rhythm with the daily rhythm of light stimulation via the LED, while the females of the control group expressed a free running laying rhythm. During phase 3, all the females were free running, so they did not synchronize their laying rhythm on the daily rhythm of visits. The slight non-significant difference between the mean values of the circadian periods for the females of the two groups (Fig. 3) can be explained by the inter-individual variability of the free running period, which can range from 24.8 to 26 h between females.
3.2 Laying time
Between phases 1 (LD) and 2 (LED), four out of five females of the treated group did not significantly change their laying time (Wilcoxon, p>0.05) (Fig. 4). One female delayed her laying time by 1.7 h (Wilcoxon, p=0.008). We noticed that this female showed one of the latest laying times in phase 1. No relationship was observed between the value of the laying time in LD and the value of the free running period in DD.
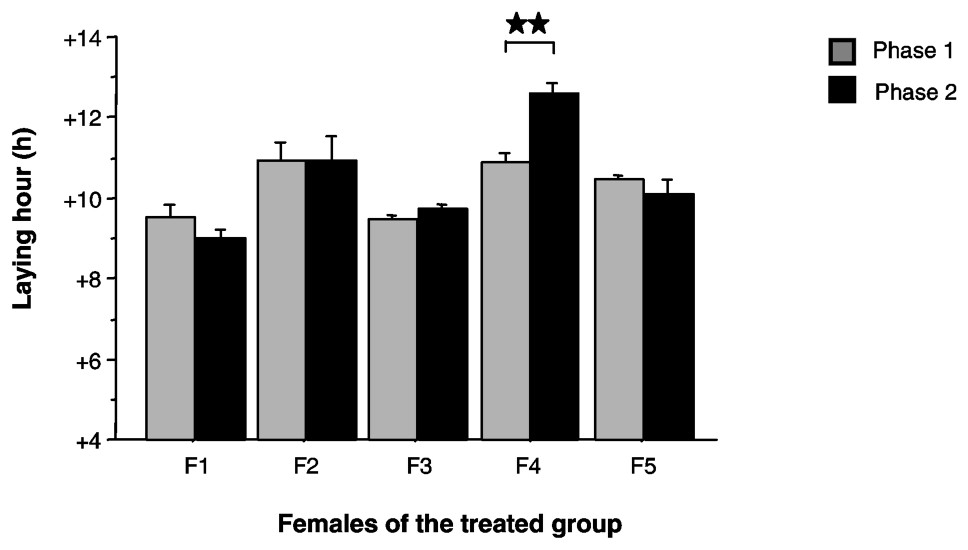
Mean laying time (+ES), defined in accordance with ‘light-on’ time, for the five females of the treated group (F1 to F5) during phases 1 and 2. : Wilcoxon, p<0.01.
4 Discussion
Thus, under constant darkness, a light/dark cycle-type stimulation, with the light directed only on the bird's cranium, can synchronize the laying rhythm of Japanese quail. In this species, it seems that intracranial receptors can transmit the photic information to the oscillator(s) controlling the ovulation/oviposition rhythm. This photic information seems necessary and sufficient to synchronize laying behaviour. So, these results are similar to those found in standard conditions of alternation of day and night with enucleated or patched females [18] and this experimental protocol seems less stressful than using blinding.
For this experiment, we did not pinealectomise the birds. On the one hand, multiple sources of melatonin secretion other than the pineal gland are well known especially the retina in Japanese quail (33% of the total secretion of melatonin) [12]. On the other hand, we demonstrated in a recent study that melatonin does not play a role in the control of the laying rhythm [17]. Given this, we can postulate that the pineal photoreceptors probably do not play an important part, if any, in the synchronization of the laying rhythm with the photoperiodic conditions. Perhaps the photoreceptors implicated could belong to those recently discovered by Yoshikawa and Oishi [19] in the paraventricular organ of the hypothalamus.
One might justifiably consider that twice visits a day and their associated manipulations could be important events. But results showed that females did not synchronized their laying rhythm with this recurrence. In Phase 3, in DD without rhythmic activity of the LED, the fact is that no clear different phases (like light/dark) were obvious for the bird, only important but short regular events. Another experiment with only short switching-on of the LED, twice a day, could let us compare the synchronizing power of light and that of human disturbance.
The variation of the laying time for one female is more difficult to explain. In previous experiments, we observed some change like this when agonistic encounters between females were forced. But behavioural dominance was not recorded here.
In conclusion, it would seem that the ocular photoreceptors are not necessary in the transmission of light information to the oscillators regulating the laying rhythm in quail.


