Version française abrégée
Le climat méditerranéen est caractérisé par des périodes de sécheresse erratiques imprévisibles, ce qui limite considérablement les productions végétales et celle des céréales en particulier. Alors que la restriction de l'eau utilisable pour l'agriculture est de plus en plus importante, la sélection de plantes tolérantes à la sécheresse apparaît comme l'une des solutions efficaces pour atténuer l'impact de cette contrainte. Cette stratégie repose sur les connaissances des mécanismes impliqués dans la tolérance à la sécheresse. Le développement des racines est considéré comme un des facteurs contribuant à la tolérance à la sécheresse. Cependant, l'étude de la morphologie racinaire est confrontée à la difficulté de travail sur les racines et à l'absence d'outils simples et rapides à utiliser sur de larges effectifs de plantes. Cette difficulté a limité le nombre de travaux sur la variabilité génétique des caractères racinaires. Néanmoins, les études menées sur les racines adventives ont montré l'existence de différences génotypiques chez les graminées. Les expérimentations réalisées sur les racines séminales sont encore plus rares. Malgré l'importance de ces dernières dans l'implantation de la jeune plante et ses conséquences sur le rendement de la culture, elles ont suscité peu d'intérêt.
En effet, le système racinaire séminal joue un rôle déterminant dans la nutrition hydrique et minérale des plantes à des stades précoces, au cours desquels la plante s'organise pour assurer une morphogenèse des racines adventives et des talles et, par conséquent, de la production. Une première étude réalisée chez l'orge a montré l'effet du stress sur le développement des racines séminales. Cependant, il est bien connu que l'orge est la céréale méditerranéenne la plus tolérante à la sécheresse, comparativement au blé dur. Par ailleurs, l'étude sur l'orge s'est focalisée seulement sur la morphologie des racines. L'examen de l'effet de stress hydrique sur les dimensions cellulaires sous conditions de stress hydrique n'a pas été abordé.
L'objectif de notre travail est d'étudier l'influence de différents régimes hydriques sur l'élongation des racines séminales et d'apporter des informations sur leur formation chez le blé dur. L'impact du stress hydrique sur les dimensions des cellules de l'assise pilifère est examiné. Nos résultats sur les racines sont comparés à ceux d'autres études sur des caractères morphologiques et physiologiques, sur les génotypes communs.
À ce titre, une expérimentation a été réalisée sur huit génotypes de blé dur cultivés en serre en conditions contrôlées à l'université de Tiaret (Algérie). Les plantes ont été installées dans des cylindres en PVC de 30 cm de diamètre et de 130 cm de profondeur, remplis d'un mélange de sable, terre et matière organique à des proportions respectives de . Quatre traitements hydriques ont été appliqués : plantes conduites à la capacité au champ (100% CC), plantes conduites sous déficit hydrique constant de l'installation à la date des mesures à des taux de 75, 50 et 25% CC. L'humidité du substrat a également été évaluée à trois profondeurs à la fin de l'expérimentation, afin de vérifier les différences de teneur en eau dans le sol. Au stade 4 feuilles bien développées, les plantes ont été récoltées et les caractères racinaires mesurés. La longueur et le poids sec des racines séminales et de la partie aérienne ont été mesurés. Le rapport des poids secs racinaire et aérien a été calculé. Le volume a quant à lui été évalué sur trois profondeurs du sol (0–15, 15–30 et au-delà de 30 cm). Des échantillons de la zone pilifère des racines ont été prélevés et la dispersion des cellules réalisée afin de déterminer la taille des cellules. La prolifération continue des racines nécessite des mécanismes adaptatifs aux niveaux cellulaire et tissulaire. L'expansion et l'élongation cellulaires sont parmi les processus les plus sensibles chez les plantes affectées par la sécheresse. L'assise pilifère est à l'interface entre la demande de la plante et la disponibilité en eau dans le milieu extérieur. De ce fait, elle est une des premières parties de la plante à réagir et à ajuster l'expansion et l'élongation de ces cellules en fonction de la contrainte hydrique. La mesure de la taille des cellules de cette assise informe sur l'impact du stress hydrique sur le développement des racines.
Les résultats obtenus montrent que les traitements hydriques appliqués ont provoqué des modifications importantes de la plupart des caractères étudiés. Des réactions contrastées ont été observées chez les génotypes testés. Une interaction génotype × traitements hydriques significative a été notée. La longueur des racines séminales est significativement plus faible sous stress hydrique sévère (25% CC) par rapport au traitement contrôle (100% CC). À l'inverse, et même si les différences ne sont pas statistiquement significatives, les racines séminales sont plus longues sous stress hydriques faible (75% CC) et modéré (50% CC). Il semblerait que les stress hydriques faible et modéré stimulent l'élongation racinaire. Le volume racinaire et sa distribution dans les différentes couches du sol présente les mêmes variations que celles de la longueur. En effet, le volume des racines augmente dans la couche superficielle (0–15 cm) du substrat avec l'intensité du stress. Inversement, la couche la plus profonde (au-delà de 30 cm) du substrat est plus faiblement colonisée par les racines au fur et à mesure que le stress devient sévère. Le rapport des matières sèches racinaire et aérienne augmente chez les plantes les plus stressées. Cette augmentation est le résultat de la chute de la quantité de matière sèche aérienne produite plutôt que de celle des racines. L'examen des cellules de l'assise pilifère montre que les dimensions de celles-ci sont d'autant plus petites que le stress subi par la plante est sévère.
D'une manière générale, les plantes cultivées sous un stress hydrique sévère présentent des racines moins longues et colonisent les couches superficielles du sol comparativement à celles des autres traitements. La croissance des organes résulte de la succession de deux phénomènes, la multiplication et l'élongation des cellules. Dans notre travail, les résultats montrent que le stress provoque une diminution des dimensions des cellules de l'assise pilifère. Cependant, cette diminution n'explique pas, à elle seule, les faibles valeurs notées pour la longueur des racines. La diminution de la vitesse de division cellulaire, voire la mort des apex, contribuerait également aux observations faites dans notre étude, surtout sous contrainte hydrique sévère. Par ailleurs, des valeurs supérieures pour la plupart des caractères racinaires ont été observées pour les traitements 75 et 50% FC par rapport à 100% FC. Il semblerait que le stress faible ou modéré stimule l'élongation et l'expansion cellulaires au niveau des racines. Nos résultats montrent que l'impact du stress hydrique sur les caractères des racines séminales est dépendant de son intensité.
Des réponses génotypiques contrastées ont été notées. Les différences les plus marquées ont été observées entre Cham 1 (génotype sélectionné dans les conditions du Moyen-Orient) et Oued Zenati et Hedba 3 (deux populations locales algériennes). Cham 1 maintient la longueur de son système racinaire séminal quelle que soit la contrainte hydrique. De même, les différentes contraintes semblent affecter fortement la production des matières sèches aérienne et racinaire chez les deux génotypes algériens. Au contraire, Cham 1 semble subir très faiblement les effets des traitements hydriques. Les études menées sur les racines adventives du blé dur à l'anthèse ont montré l'existence de différences de profondeur d'enracinement et de réponse à la contrainte. Elles rejoignent celles déjà réalisées sur des caractères morpho-physiologiques foliaires sous déficit hydrique. Il apparaît donc que Cham 1 présente un spectre d'adaptation large, qui lui permet de tolérer différentes conditions hydriques, ce qui lui confère une stabilité de rendement. Au contraire, la tolérance des génotypes algériens serait de type local.
En résumé, le développement des racines séminales dépend de la sévérité du stress hydrique imposé aux plantes. Un stress hydrique sévère réduit la longueur et le volume des racines séminales, principalement dans les couches profondes du sol. Cette réduction est due à la diminution de la taille des cellules dans les racines, mais probablement aussi à la réduction de la vitesse de la division cellulaire, voire son arrêt. Une large variabilité génotypique a été observée dans cette étude. Cette différence est marquée entre les génotypes moyen-orientaux, qui développent des racines profondes, y compris sous une forte contrainte hydriques, et les génotypes algériens, qui exhibent un enracinement superficiel sous stress hydrique sévère. Néanmoins, une étude sur une collection plus large permettrait de conforter les différences observées entre les génotypes du Moyen-Orient et les génotypes algériens. L'examen anatomique des racines demande à être approfondi et élargi à l'étude de la réaction de la division et de l'élongation cellulaires au déficit hydrique.
1 Introduction
Drought is the most abiotic stress factor limiting crop productivity. This is particularly true in the Mediterranean region. In this area, the climate is characterised by erratic and unpredictable precipitations. As water available for agricultural uses becomes more restrictive, the release of drought-tolerant genotypes will become increasingly important [1,2]. The understanding of the physiological and the genetic adaptive mechanisms controlling drought tolerance is a crucial aspect in plant biology. The response of root growth to water deficit conditions is one aspect contributing greatly to drought tolerance. However, root morphology studies suffered from the difficulty in working with roots and from the absence of simple and fast tools to evaluate root traits on a large number of plants. Consequently, studies of genetic variability of root characteristics are scarce. Most of the experiments have been done on adventitious roots for which differences between cultivars have been observed in some cereals and grasses [3–10]. Few others studies have concerned seminal roots in bread wheat [6,11], barley [2], and oat [12]. More recently, the effect of water deficit on barley seminal roots was studied [13]. These authors have shown that severe water deficit strongly reduces seminal root growth in deeper soil layers. This study has provided interesting results for an understanding of seminal root responses under water deficit, but it was only focussed on morphological traits. The effect of stress on cell size was not approached. Besides, it is well known that barley is more drought-tolerant than other Mediterranean cereals [2,3]. Drought acts on barley yield mostly through the number of tillers by plant. In contrast, the number of grains by ear is the most important yield component in durum wheat. This latter species is confined to arid and semi-arid areas in Mediterranean countries. Historically, durum wheat has received insufficient attention from breeders. Farmers, even in low rainfall zones, prefer bread wheat, which relegates durum wheat cultivation to more marginal areas. Despite its importance for human diet, little progress has been made for durum wheat yield improvement in dry areas.
The experiments performed on durum wheat under various water conditions have shown genotypic difference for adventitious root growth [14–16]. Investigations on seminal roots in durum wheat are lacking. Even if genotypic differences exist during later growth stages for root traits, it is not sure that such differences exist at early stages, especially when unexpected drought periods occur. Moreover, physiological studies on durum wheat have been fairly done on local germplasm and results obtained in different works could not be compared.
Our study focuses on the harmful effects of water deficit on durum wheat seminal root growth. The pattern of seminal root growth is investigated at various levels of soil water content and depth. The impact of water deficit on cell size is prospected. The genotypic responses for the traits measured in our study are compared to those obtained for other physiological characters collected under controlled conditions or in field-grown experiments.
2 Material and methods
2.1 Plant material
Eight durum wheat genotypes (Triticum durum Desf.) from different geographic origins and differing in response to drought were chosen for this study (Table 1). They include two Algerian landraces, four accessions from the Italian germplasm and two cultivars from the Arabian Centre for Studies of Arid Zones and Drylands (ACSAD) and from the International Centre for Agricultural Research in Dry Areas (ICARDA). Little information is available on Italian accessions and on ACSAD 299. Algerian landraces are known as locally adapted and are characterized by low productivity and low yield stability. Cham 1 is a drought-tolerant improved line presenting high yield stability.
List of the durum wheat genotypes used in this study
| Genotype | Type | Origin |
| Neffer | Advanced line | IAO (Italy) |
| ACSAD 299 | Advanced line | ACSAD (Syria) |
| Oued Zenati | Landrace | Algeria |
| CTA 264 | Advanced line | IAO (Italy) |
| Hedba 3 | Landrace | Algeria |
| Jappa 3246 | Advanced line | IAO (Italy) |
| Appulo | Advanced line | IAO (Italy) |
| Cham 1 | Advanced line | ICARDA (Syria) |
2.2 Experimental design and water conditions
The experiment was conducted under glasshouse-controlled conditions at the University of Tiaret (Algeria). Diurnal and nocturnal temperatures were maintained at 20 °C and 15 °C, respectively. The relative humidity was 70%. The photoperiod was maintained at 15 hours/day, with a supplement of light of 85 W m−2.
A randomized complete block design was used with two blocks. In each block, water treatments were repeated three times. Each genotype was represented by nine plants in each water treatment within each block.
Seeds were put in Petri dishes for germination. After the emergence of the first leaf, the seedlings were grown in PVC cylinders (130-cm height and 30-cm diameter) filled with a mixture of sand, soil and organic dry matter (8:1:1). After complete emergence of the second leaf, different water treatments were applied. Plant controls were conducted at the field capacity (100% FC). For the three other treatments, plants received 75, 50 and 25% FC, respectively. The water stress was applied until four full expanded leaves. The studied genotypes were characterized by phenological differences, mostly at anthesis. These genotypes did not present any differences in phenology at early stages and were, therefore, subjected to equal stress duration periods.
2.3 Measurements
2.3.1 Root traits
Roots were harvested at four full expanded leaves, and were washed in order to eliminate the potting mixture residue. In each water treatment and for each genotype, measurements were performed on nine plants. The length of roots (in cm) was measured. Seminal root volume was evaluated at three depth layers (0–15 cm, 15–30 cm and more than 30 cm) by immersion in a graduate test tube and measure of the displaced water volume. Root samples and aerial fresh samples were oven-dried at 80 °C for 48 h and weighed to determine the dry matter weight of each part. The ratio of root-to-shoot dry matters (RDM/SDM) was then calculated.
The preparation of the tissue samples was done according to Rascio et al. [17]. In order to analyse the cell size, a 1-cm section was cut from root piliferous part. The external zone (piliferous layer and external part of cortical parenchyma) was delicately isolated and fixed in ethanol:formalin: acetic acid (17:3:1). The fixed tissue was immersed in 6 N NaOH for 36 h to obtain isolated cells, and then rinsed in water and on a glass slide in one drop of 0.1% aqueous safranin. Size assessments concerned only piliferous cell. They were determined by micrometer of a microscope (Zeiss, Germany) and were transformed in micrometres with utilisation of micrometric coefficient (×2.33).
2.3.2 Soil water content
After plant harvest, soil samples were collected in order to evaluate accurately the soil water content (SWC). Soil samples were weighed and reweighed after drying at 105 °C during 24 h. SWC was determined at 0–15-cm, 15–30-cm and beyond 30-cm depth. The measurements were done on five samples for each depth in each water treatment.
2.4 Statistical analyses
All data were subjected to analysis of variance using the GLM procedure of SAS (SAS Institute, 1987, Cary, NC, USA). Comparisons between water treatments and between genotypes, within each water treatment, were based on the Duncan test at 5% probability level.
3 Results
Table 2 summarizes the soil water content (SWC) values obtained for 15-cm depth increments in each water treatment. A significant difference in SWC was noted between treatments. The distribution of water in different soil layers was different among treatments. The proportion of water located in the upper soil layer (0–30 cm) increased with the severity of the applied stress. Under control conditions, 60% of SWC was localized in the upper soil layer (0–30 cm). Under 25% FC, 90% of SWC was located in the top soil layer.
Soil water content (%) at three depths in each water treatment. Measurements were made on nine samples for each depth in each water condition at the end of experiments
| Water treatment (% of field capacity) | 25% FC | 50% FC | 75% FC | 100% FC | ||||
| Depth | Mean | SE | Mean | SE | Mean | SE | Mean | SE |
| 0–15 cm | 2.94 | 0.44 | 19.44 | 0.48 | 21.09 | 0.37 | 25.90 | 0.76 |
| 15–30 cm | 1.14 | 0.01 | 12.30 | 0.20 | 19.24 | 0.17 | 38.53 | 0.30 |
| Beyond 30 cm | 0.00 | 0.00 | 3.39 | 0.45 | 4.17 | 0.59 | 29.30 | 0.83 |
There were significant differences between applied water treatments and between the studied genotypes for all measured traits. The genotype by water treatment interaction was also significant for all characters (Table 3).
Mean square values and the significance of water treatment, genotype and their interaction effects on root traits measured on eight durum wheat genotypes grown under four water treatments. Degrees of freedom (d.f.) are also displayed
| Trait | Water treatment effect (d.f. = 3) | Genotype effect (d.f. = 7) | Genotype × water treatment effect (d.f. = 21) |
| Seminal root length | 1818.36⁎⁎⁎ | 109.94⁎⁎⁎ | 103.30⁎⁎⁎ |
| Root to shoot dry matters ratio | 0.1037⁎⁎⁎ | 0.0033⁎⁎⁎ | 0.0025⁎⁎⁎ |
| Seminal root volume | |||
| 0–15 cm layer | 0.48⁎⁎⁎ | 0.06⁎⁎⁎ | 0.03⁎⁎⁎ |
| 15–30 cm layer | 0.15⁎⁎⁎ | 0.04⁎⁎⁎ | 0.04⁎⁎⁎ |
| beyond 30 cm | 0.26⁎⁎⁎ | 0.05⁎⁎⁎ | 0.03⁎⁎⁎ |
| Piliferous layer | |||
| cell length | 444.57⁎⁎⁎ | 1453.39⁎⁎⁎ | 134.51⁎⁎⁎ |
| cell width | 183.46⁎⁎⁎ | 59.10⁎⁎⁎ | 18.64⁎⁎⁎ |
⁎⁎⁎ Significant at .
Water treatments have significantly affected the measured traits. Genotypic averaged value of seminal root length at 100% FC was 55.6 cm. This value was significantly lower when soil water content went below 50% FC. Seminal roots were 34% shorter under severe water stress than under control conditions (Fig. 1a).
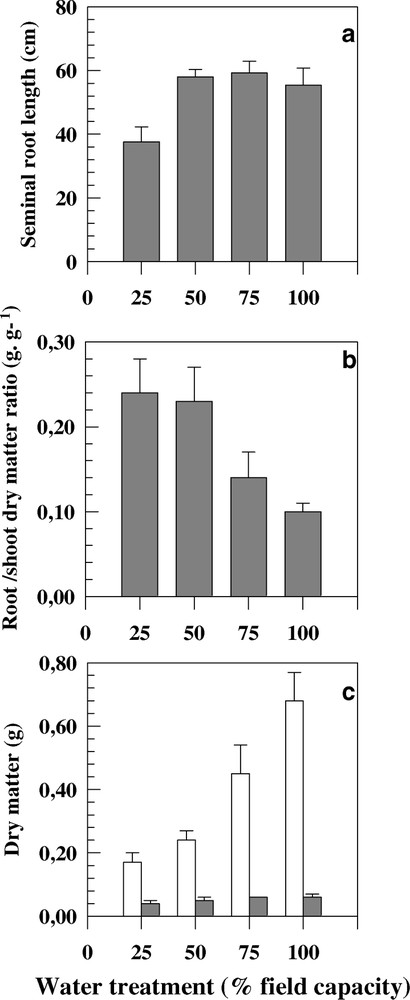
Seminal root length (a), root-to-shoot dry matter ratio (b), and root and shoot dry matters (c) measured on eight durum wheat genotypes cultivated under four water treatments. Bars represent the standard error.
The seminal root volume was measured in three soil layers (0–15 cm, 15–30 cm and beyond 30 cm) for each water treatment. Globally stressed plants exhibited significantly higher root volume values (Table 4). The distribution of the root volume through soil layers was different between water treatments. For 100%, 75% and 50% FC, three quarters of the root volume were localised in the upper layers (0–30 cm). Under severe water stress, nearly 94% of roots were confined in the top soil layer (Table 4). At the top soil layer (0–15 cm) and under severe water stress, root volume was two times higher than under control conditions. In the second soil layer, root volume was significantly higher under both low and moderate water deficit compared to plants under 25 and 100% FC treatments (Table 4). Beyond 30-cm depth, root volume was slightly low for 75 and 50% FC treatments compared to control plants. In contrast, the root volume values fell in below 50% FC (Table 4).
Mean values and standard error (SE) of length and width of piliferous layer cells and root volume at 3 different depths measured on eight durum wheat genotypes grown under four water treatments. Means indicated by different letters (within each water treatment) are significantly different (at 0.05 probability level) by the Duncan comparison test
| Water treatment (in % field capacity) | 25% | 50% | 75% | 100% | LSD | ||||
| Trait | Mean | SE | Mean | SE | Mean | SE | |||
| Cell length (μm) | 51.6B | 9.2 | 53.0B | 10.4 | 58.8A | 14.3 | 60.7A | 20.2 | 2.4 |
| Cell width (μm) | 21.7C | 2.3 | 22.5C | 3.4 | 24.7B | 3.4 | 28.0A | 3.1 | 1.3 |
| Seminal root volume (cm3) | |||||||||
| 0–15 cm layer | 0.64A | 0.15 | 0.41B | 0.10 | 0.36C | 0.06 | 0.32D | 0.09 | 0.01 |
| 15–30 cm layer | 0.28B | 0.13 | 0.43A | 0.10 | 0.42A | 0.07 | 0.30B | 0.07 | 0.02 |
| beyond 30 cm | 0.06D | 0.07 | 0.25B | 0.13 | 0.40A | 0.13 | 0.19C | 0.10 | 0.01 |
| total volume | 0.98B | 0.18 | 1.09A | 0.17 | 1.08A | 0.16 | 0.81C | 0.19 | 0.02 |
RDM/SDM was higher when the soil water content decreased. This trait was 2.5 times lower in control plants than in severely stressed plants (Fig. 1b).
Water shortage has affected significantly piliferous cell sizes, especially plants under 50 and 25% FC treatments, which have presented lower cell size compared to the other water treatments (Table 4).
Genotypic differences were large for all measured traits. For both root length and volume, globally and as described before, a slight increase in traits values was noted, followed by a marked decrease when the soil water was below 50% FC (Tables 5 and 6). However, the genotypes did not respond similarly to water shortage. While Jappa, Hedba 3 and Oued Zenati exhibited the longest seminal roots under 100% FC, Neffer, Appulo and CTA presented the shortest ones under severe water stress. The root length of Cham 1 and, at a lesser extent, of ACSAD 299, was slightly influenced whatever the stress intensity. Similar responses were observed for root length and RDM/SDM. The latter trait was more than three times higher under severe water stress compared to 100% FC for Hedba 3 (Algerian landrace). The smallest difference between extreme water treatments for RDM/SDM was noticed for the Middle-East genotype ACSAD 299 (Table 5). CTA and Cham 1 increased their root volume under 75 and 50% FC and maintained it even under severe water conditions compared to the six other accessions. The distribution of roots through the soil profile was different between genotypes. Cham 1 developed important root volume beyond 30-cm depth. In contrast, Algerian and Italian (except CTA) accessions did not develop roots beyond 30 cm depth. For cell sizes, six genotypes presented similar pattern of response to water shortage. They exhibited smaller cells under stressed conditions, whereas Cham 1 and CTA maintained cell sizes whatever the water treatment (data not shown).
Seminal root length and root-to-shoot dry matters ratio measured on eight durum wheat genotypes grown under four water treatments. Means indicated by different letters (within each water treatment) are significantly different (at 0.05 probability level) by the Duncan comparison test
| Trait | Seminal root length (cm) | Root to shoot dry matter ratio (g g−1) | ||||||
| Water treatment (% of field capacity) | 25% | 50% | 75% | 100% | 25% | 50% | 75% | 100% |
| Genotype | ||||||||
| Neffer | 27.0E | 58.0A | 60.3AB | 37.0E | 0.237C | 0.234B | 0.206A | 0.098B |
| ACSAD 299 | 41.5B | 60.8A | 61.4AB | 49.5D | 0.208DE | 0.172D | 0.164B | 0.119A |
| Oued Zenati | 37.2C | 57.8A | 56.0C | 62.3A | 0.262B | 0.194C | 0.141C | 0.099B |
| CTA 264 | 35.5C | 59.0A | 58.8B | 60.3B | 0.221CD | 0.196C | 0.127D | 0.099B |
| Hedba 3 | 41.7B | 58.7A | 61.2AB | 62.5A | 0.305A | 0.265A | 0.131CD | 0.092C |
| Jappa 3246 | 36.0C | 58.2A | 61.2AB | 63.8A | 0.204DE | 0.234B | 0.124DE | 0.090C |
| Appulo | 31.3D | 53.0B | 62.5A | 54.7C | 0.198E | 0.232B | 0.113E | 0.090C |
| Cham 1 | 50.7A | 60.0A | 61.2AB | 49.2D | 0.238C | 0.236B | 0.123DE | 0.085D |
| LSD | 2.8 | 2.7 | 2.8 | 1.5 | 0.020 | 0.020 | 0.011 | 0.005 |
Mean values of seminal roots volume (cm3) measured at three soil depths on eight durum wheat genotypes grown under different water treatments. Means indicated by different letters (in each soil layer and within each water treatment) are significantly different (at 0.05 probability level) by the Duncan comparison test
| Water treatment (in % field capacity) | 25% | 50% | 75% | 100% | ||||||||
| Soil layer (cm) | 0–15 cm | 15–30 cm | Beyond 30 cm | 0–15 cm | 15–30 cm | Beyond 30 cm | 0–15 cm | 15–30 cm | Beyond 30 cm | 0–15 cm | 15–30 cm | Beyond 30 cm |
| Genotype | ||||||||||||
| Neffer | 0.49E | 0.17E | 0.01C | 0.21F | 0.32B | 0.12DE | 0.27E | 0.43C | 0.15G | 0.27D | 0.35B | 0.08D |
| ACSAD 299 | 0.68C | 0.05F | 0.05B | 0.46C | 0.53A | 0.27C | 0.45A | 0.40D | 0.27D | 0.50A | 0.25D | 0.14C |
| Oued Zenati | 0.72C | 0.23D | 0.03B | 0.38E | 0.52A | 0.27C | 0.43A | 0.30F | 0.21E | 0.42B | 0.33B | 0.23B |
| CTA 264 | 0.95A | 0.22D | 0.04B | 0.38E | 0.28B | 0.27C | 0.32D | 0.40D | 0.32C | 0.30C | 0.23D | 0.12CD |
| Hedba 3 | 0.48E | 0.15E | 0.05B | 0.38E | 0.50A | 0.48A | 0.37B | 0.53A | 0.33C | 0.24E | 0.34B | 0.13C |
| Jappa 3246 | 0.43F | 0.43C | 0.05B | 0.50B | 0.47A | 0.15D | 0.36BC | 0.45C | 0.18F | 0.31C | 0.28C | 0.27B |
| Appulo | 0.77B | 0.53A | 0.00C | 0.41D | 0.33B | 0.10E | 0.34BCD | 0.50B | 0.57A | 0.31C | 0.42A | 0.38A |
| Cham 1 | 0.57D | 0.47B | 0.23A | 0.53A | 0.50A | 0.37B | 0.33CD | 0.37E | 0.38B | 0.22F | 0.18E | 0.13C |
| LSD | 0.04 | 0.03 | 0.03 | 0.02 | 0.09 | 0.04 | 0.03 | 0.03 | 0.03 | 0.01 | 0.03 | 0.03 |
4 Discussion
The measured traits were significantly affected by water stress and its severity. The ranking of genotypes was different depending on stress intensity (Fig. 1, Tables 5 and 6). Water treatment by genotype interaction mirrored differential responses to increasing water stress. The incidence of the cross-over interaction emphasizes that root attributes cannot be predicted from results obtained under non-stressed conditions. Therefore, some authors have recommended the screening and selection of drought-tolerant genotypes under stressed conditions [2,18].
Seminal roots length was affected by the decrease in the soil water content. The roots were significantly shorter under severe water stress conditions (Fig. 1a). Similar results have been observed on rice [8] and barley [13] seminal roots. Contrasted results have been reported on adventitious root length in response to water deficit on cereals. Water stress may promote longer roots in durum wheat [14,15] and maize [19]. On the contrary, shorter roots were shown in water stressed barley [7] and wheat [5,20]. In fact, the most important difference between the two groups of studies was the intensity of the imposed water stress. In the first works [14,15,19], soil water content was between 60 and 70% FC. In contrast, in barley and wheat reports [5,20], plants have grown on soil containing less than 50% FC. Barley seminal root traits were significantly lower, particularly under severe water shortage [13]. In our study, even not statistically significant, low and moderate water stress (75 and 50% FC) showed higher values for root length compared to 100% FC. This result was expected, since similar behaviours have been reported in seminal and adventitious roots of several cereals [7,8,10,14,15]. It seems that low levels of water stress promote root length by stimulating cell expansion and elongation [19]. Inversely, severe water treatment (25% FC) inhibited root growth and then reduced their length (Fig. 1a). These results emphasize the importance of water stress intensity on the expression on root length.
The root volume was also strongly affected by water deficit (Table 4). The seminal root repartition was found closely related to water availability in the different soil layers. Root volume was not significantly affected in top soil layer, but was strongly reduced in deeper layers (Table 4). Under severe water stress, there were no roots in the deepest soil layer. More than 90% of the roots were confined in the top soil layer (Table 4). This is probably associated with high extractible soil water [7]. Most of the available water was localized in the upper part of the soil under severe water conditions. Low and moderate water treatments slightly affected root volume in top and middle soil layer, which was expected (Table 4). Previous studies conducted on bread wheat and barley grown under stressed conditions have reported that 60 to 90% of roots were confined in surface layers of the soil [6,13,21].
Many works have reported the effect of stress duration on adventitious roots [14,22], or have demonstrated that the seedling stage when water deficit occurs is more important than the duration of the stress [11,16]. Other authors have noticed that root growth is dependent on both stress severity and timing [13,23]. The seminal roots sensitivity to water stress is likely due to the absence of a cuticle that could protect them against water evaporation [9]. Root systems are responsible for water and nutrients uptake. Therefore, the great influence of environmental conditions on root system growth is not surprising [24]. Root growth depends on both cell elongation and expansion, meristem activity and growing conditions prevailing during these phenomena [19,24]. Some reports have underlined the extreme sensitivity of cell expansion to water deficit [9,17]. In our study, the applied water treatments have significantly affected cell size of the piliferous layer (Table 4). Smaller cells may explain shorter roots under severe water deficit. Piliferous layer cells, as other root tissues cells, divert from meristematic ones. The size of the former cells is dependent on water conditions occurring during elongation and differentiation process of meristematic cells. Under well-watered conditions, the elongation zone of primary roots in maize encloses the apical 12 mm. Cells are produced in the meristem and are displaced basally by production and expansion of new cells. In water stressed roots, cell elongation is preferentially maintained towards the apex [19]. The relative elongation rate in maize primary roots is progressively inhibited when moderate water stress is reached [19]. However, the decrease in cell size is not the only mechanism involved in response to water shortage. The absence of seminal roots in the deeper soil layers under severe water deficit may result from the death of apices and from the non-initiation of new lateral roots [4,11,24].
Significant differences were noticed between water treatments for RDM/SDM. Values of this trait increased from 100% FC treatment to severe water deficit (Table 3 and Fig. 1b). RDM/SDM has been measured in several studies and frequently an increase of this ratio was noted under water stress conditions [10,13,16,17,20,22]. In our study, seminal roots have maintained elongation rates at low soil water content, whereas shoot growth was inhibited as presented in Fig. 1c. In plants growing under dry conditions, root system development is usually less inhibited than shoot growth. Maintenance of root growth during water deficit is obviously important to maintain an adequate plant water supply. In young maize plant, it has been shown that leaf elongation was significantly inhibited by water deficit, contrary to root elongation, which was unaffected. The competition between transpiration and leaf growth rather root growth may explain the contrasted responses of the two plant parts to water deficit [9,24].
A broad genotypic variation was observed for all the studied root traits. The scarce studies done in durum wheat on adventitious roots under water stress have also reported genotypic variability [14,15]. The Middle-East advanced line Cham 1 exhibited different behaviour compared to the Algerian landraces Oued Zenati and Hedba 3 (Tables 5 and 6). Previous studies have noticed deeper adventitious roots at anthesis for Cham 1 than for the both Algerian landraces [15]. In this latter study, water deficit has affected greatly RDM (45% of reduction compared to control plants) and SDM (50%) in Oued Zenati and Hedba 3. The three genotypes also differed for osmotic adjustment capacity and water status parameters [14,15,25]. Water stress have caused about 25% and 10% of reduction of leaf relative water content from favourable to stressed conditions in the Algerian genotypes and in Cham 1, respectively. Other leaf physiological traits were studied in these accessions. Flag leaf carbon isotope discrimination and ash content were shown high under drought conditions in Cham 1 compared to both Hedba 3 and Oued Zenati [18]. This difference is probably due to the high stomatal conductance of Cham 1, which permits transpiration even under water deficit [26]. Accumulation of various osmoticum (as soluble carbohydrates) seems more active in Cham 1 than in the Algerian landraces, which results in higher osmotic adjustment capacity [25]. This latter trait allows us to maintain high cell turgor inducing great transpiration rate in Cham 1. All these studies have underlined the wide drought tolerance and yield stability of Cham 1 and the specific adaptation of Oued Zenati and Hedba 3 to Algerian conditions. The observed differences for seminal roots as well as for aerial physiological traits in these genotypes contribute probably to their contrasted behaviour under drought.
In summary, in this study, genotypic differences were observed for seminal roots traits in durum wheat. A differential impact of water stress on roots was noted. Seminal root characters were affected by water deficit and its impact is related to its intensity. Severe water deficit inhibited seminal root growth in deeper soil layers. An anatomical approach has shown that water deficit reduced the size of piliferous layer cells, which could partly explain the limitation of root growth in deeper soil layers. It also seems that the severe water stress may cause the death of apices limiting root growth. A differential response to water deficit was observed mainly between Middle-East and Algerian genotypes, confirming the previous reports on adventitious roots and on leaf physiological traits. These differences need to be deepened by study of a large collection of genotypes. Moreover, anatomical changes in different root tissues as well as the impact of drought on cell elongation and expansion need further investigations. Molecular and genetic studies could help to understand the differences between these genotypes and to improve our knowledge of the mechanisms involved in seminal-root response to water deficit.


