1 Introduction
The role of taxonomic richness in ecosystems structure and function has been recognized for a long time [1–4], leading to an increased interest in habitat and species conservation in many ecosystems, including streams and rivers. Streams, like all ecosystems, are hierarchically organized, from catchment to microhabitat [5], thus taxonomic richness can be considered from regional to local scales [6–8]. At the smallest scale, taxonomic richness depends firstly on the aptitude of habitats to harbour organisms and secondly on the pool of taxa present at a larger scale and the ability of these taxa to pass through natural and anthropogenic filters before reaching the local community [9]. In streams, bed substratum and hydraulic conditions provide a wide array of habitat conditions for invertebrates, which contribute to large-scale biodiversity.
Human activities (land use, agriculture, and urbanization) generally operate at reach and catchment scales, but alter stream habitat locally through a cascade of physical and chemical changes (temperature increase, inputs of organic and inorganic nutrients, siltation, etc.) [10]. In the Mediterranean area, human activities impacts on aquatic communities are particularly crucial, since fast human population growth is leading to highly urbanized stream catchments where water demands and pollution inputs increase [11–16]. This is not exclusive to the Mediterranean area, but it is more common there than in other areas, because the Mediterranean climate provides harsh conditions for aquatic life. Hydrological regime is characterized by strong seasonal variability with flashy flood events during spring and autumn rainfall, and low flow conditions or even drought for long periods in summer [13,17,18]. Hence, the combination of human impact and natural conditions makes the Mediterranean streams especially vulnerable to taxonomic richness loss. Further investigations of small Mediterranean streams and their tributaries are urgently required in order to establish biodiversity estimates at the catchment scale.
Considering the upper part of a catchment where the main stem is impacted by point source pollution (effluent of a wastewater treatment plant) and five non-impacted tributaries, the two following questions were addressed:
- (1) at the reach scale, what is the extent of the taxonomic richness erosion in this stream as a result of human activities?
- (2) at the catchment scale, what is the role of the perennial and intermittent flow tributaries in the conservation of benthic macroinvertebrate taxa richness?
Macrobenthic assemblages were studied with an approach based on within-habitat-type comparisons as the role of habitats on stream invertebrate communities has been clearly established.
2 Methods
2.1 Study area
The Arc is a coastal Mediterranean stream situated in southeastern France. It rises at 467 m a.s.l. and flows east–west for 85 km, draining a 780-km2 catchment area. The climate of this area is typically Mediterranean. Precipitations mainly occur as rainfall in autumn, and the summers are hot and dry. The mean annual precipitation is 683 mm [11]. Most of the tributaries dry out in summer, but some of them are artificially permanent. The upper part of the catchment (study area) is mainly agricultural, in contrast to the lower part, which is industrial. The main pollutant sources in the study area are wastewater treatment plants and diffuse agricultural pollutions.
Four stations (Arc1, Arc2, Arc3, and Arc4) were studied on an 8.3-km section of the main stem (Fig. 1). Arc1 is located upstream of the Trets wastewater processing plant with no declared point source pollutions above. The other three stations are downstream and are directly affected by the effluent. Five tributaries of the Arc were also sampled at one station each; the Aigue Vive (AI), the Aubanède (AU), the Bayeux (BA), the Cause (CA), and the La Partie (PA).
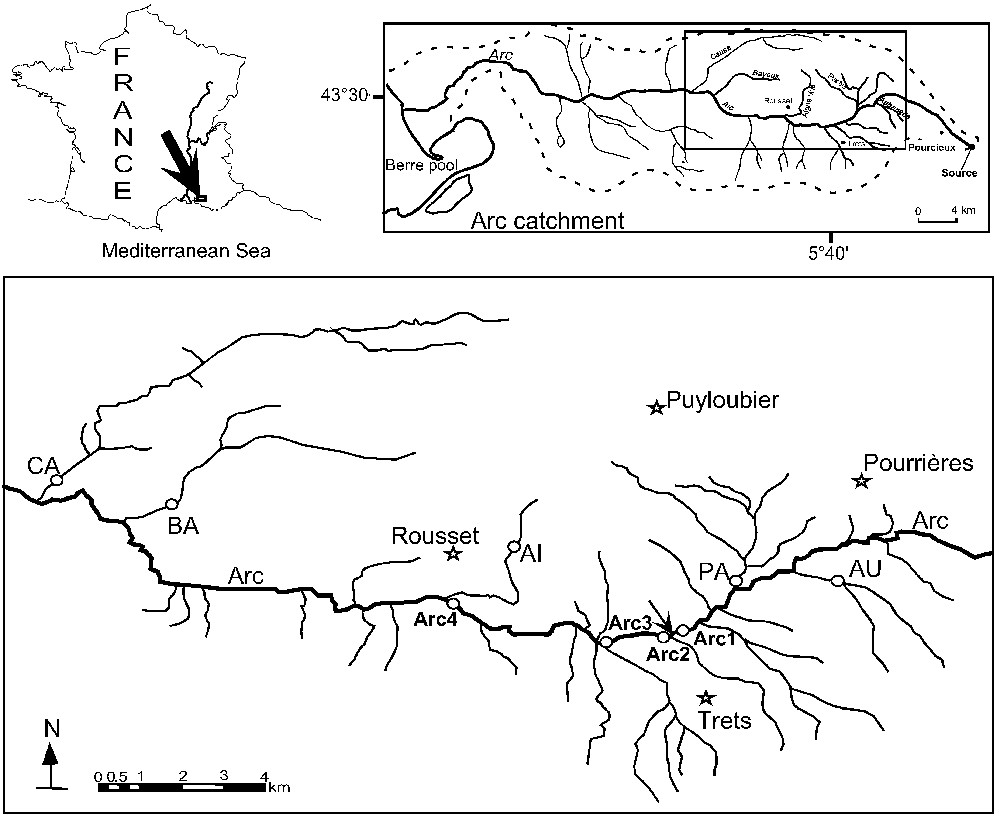
Map of the study area showing the nine study stations (four stations along the Arc stream: Arc1, Arc2, Arc3 and Arc4; and five stations on the tributaries: AU for Aubanède, PA for La partie, AI for Aigue vive, BA for Bayeux and CA for Cause). The arrow locates Trets wastewater treatment plant.
The hydrological regime at all the Arc stations and two tributaries, namely CA and BA are perennial, whereas tributaries AU, PA, and AI have an intermittent flow regime (Table 1). The conductivity values were relatively higher in the Arc stream, and in the intermittent tributaries. Water temperature and oxygen concentrations are very variable, depending, in part, on the seasonal flow regime. Low concentrations of dissolved oxygen were observed in the four Arc stations and in the AU, whereas oxygen concentration was always above 7 mg l−1 in the two perennial flow tributaries (BA and CA).
Summary of some features of the nine study stations with minimum–maximum values of water temperature, conductivity and dissolved oxygen concentrations measured between February and June 2003
| Station | Hydrology | Conductivity range (μ S cm −1 ) | Water temperature range (°C) | Dissolved oxygen range (mg l −1 ) |
| Arc1 | Perennial | 874–1071 | 4.2–22.8 | 4.3–13.1 |
| Arc2 | Perennial | 902–1045 | 4.5–22.5 | 0.9–12.0 |
| Arc3 | Perennial | 905–1074 | 4.2–20.4 | 1.6–12.2 |
| Arc4 | Perennial | 930–1028 | 4.0–20.3 | 2.3–11.7 |
| AU | Intermittent | 752–796 | 5.9–17.8 | 1.8–11.9 |
| PA | Intermittent | 963–968 | 4.2–19.0 | 5.6–12.3 |
| AI | Intermittent | 784–833 | 7.8–24.0 | 9.5–10.5 |
| BA | Perennial | 428–866 | 7.9–21.6 | 7.3–11.7 |
| CA | Perennial | 498–635 | 10.9–18.6 | 8.1–11.2 |
2.2 Sampling design and laboratory analyses
One hundred thirty-four macroinvertebrates samples (5 samples × 9 stations × 3 dates minus 1 for technical problems) were collected on three dates (February for winter, April for spring and June for summer). Benthic macroinvertebrates were sampled randomly using a Surber net (300 μm mesh size), water depth and current velocity were also measured and substratum was quantitatively described [19]. Samples were fixed using a 10% formalin solution prior to be processed in the laboratory, and the macroinvertebrates were identified to the lowest possible taxonomic level using the method of Tachet et al. [20].
2.3 Data analyses procedure
2.3.1 Taxonomic richness and spatio-temporal units at the catchment scale
A correspondence analysis (C.A.) [21] was performed on a matrix of 102 taxa × 27 station-date combinations. For one taxon, data associated with each station-date combination is the sum of the abundance of the five corresponding sampling spots. Quantitative data were transformed prior to statistical analysis to normalize and homogenize the variance. A between-class analysis was performed to compute the between-class inertia, enabling us to decompose the variance and to determine which criteria (date or station) mainly explain this variance. The variances were tested with a Monte Carlo randomization test (number of random matching = 1000) [22]. Convex hulls were drawn from a cluster analysis to make up spatio-temporal units [23].
2.3.2 Habitat typology
A Multiple Correspondence Analysis (M.C.A.) [24] was realized on three qualitative physical variables: water depth, current velocity, substratum type (most represented substratum in the sampled spot). Those three variables were represented by 13 modalities (water depth in three modalities: , , ; current velocity in 4 modalities: , , , ; and substratum type in six modalities: silt, sand, cobble-pebble, boulder, litter, roots). Based on this M.C.A., a cluster analysis was performed and different habitat types were defined. Finally, each sample out of the 134 collected samples was assigned a habitat type. All multivariate analysis were conducted using ADE-4 [25].
2.3.3 Richness patterns and faunal entities structure at the catchment scale
All the benthic samples belonging to the same habitat type spatio-temporal unit combination were grouped and treated as one entity, so-called faunal entity. Each faunal entity (A1 to C4 in Table 2) represents the assemblage of taxa in a habitat type at a spatio-temporal unit.
Shannon's diversity index (H) and Shannon's equitability (Eq) values for each of the 12 faunal entities (A, B and C are for the spatio-temporal units; 1 to 4 are for the habitat types); richness, distinctness and relative abundance values for all taxa and the most represented insect orders
| Spatio-temporal units | A | B | C | |||||||||
| Habitat types | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 |
| Faunal entities | A1 | A2 | A3 | A4 | B1 | B2 | B3 | B4 | C1 | C2 | C3 | C4 |
| Number of samples | 9 | 13 | 17 | 25 | 6 | 11 | 13 | 10 | 3 | 13 | 11 | 3 |
| H | 2.61 | 2.24 | 2.14 | 2.16 | 3.17 | 3.25 | 3.90 | 3.30 | 3.28 | 3.23 | 3.02 | 2.72 |
| Eq | 0.53 | 0.45 | 0.41 | 0.42 | 0.57 | 0.57 | 0.67 | 0.54 | 0.65 | 0.59 | 0.54 | 0.51 |
| Richness | ||||||||||||
| Plecoptera | 0 | 0 | 0 | 0 | 1 | 3 | 1 | 0 | 1 | 1 | 3 | 3 |
| Trichoptera | 1 | 3 | 6 | 4 | 5 | 6 | 10 | 8 | 5 | 7 | 8 | 3 |
| Ephemeroptera | 3 | 4 | 3 | 3 | 4 | 8 | 6 | 7 | 5 | 5 | 7 | 6 |
| Coleoptera | 5 | 5 | 4 | 1 | 7 | 7 | 7 | 14 | 3 | 7 | 6 | 6 |
| Diptera | 8 | 9 | 13 | 10 | 12 | 12 | 15 | 13 | 9 | 13 | 13 | 12 |
| All taxa (α diversity) | 29 | 30 | 37 | 35 | 45 | 49 | 57 | 66 | 32 | 45 | 48 | 38 |
| Distinctness | ||||||||||||
| Plecoptera | 0.00 | 0.00 | 0.00 | 0.00 | 50.00 | 75.00 | 25.00 | 0.00 | 50.00 | 25.00 | 75.00 | 100.00 |
| Trichoptera | 12.50 | 33.34 | 50.00 | 40.00 | 62.50 | 66.67 | 83.34 | 80.00 | 62.50 | 77.78 | 66.67 | 30.00 |
| Ephemeroptera | 37.50 | 50.00 | 42.86 | 42.86 | 50.00 | 100.00 | 85.72 | 100.00 | 62.50 | 62.50 | 100.00 | 85.72 |
| Coleoptera | 50.00 | 41.67 | 50.00 | 7.15 | 70.00 | 58.34 | 87.50 | 100.00 | 30.00 | 58.34 | 75.00 | 42.86 |
| Diptera | 57.15 | 52.95 | 61.91 | 52.64 | 85.72 | 70.59 | 71.43 | 68.43 | 64.29 | 76.48 | 61.91 | 64.00 |
| All taxa | 46.78 | 42.86 | 49.34 | 41.67 | 72.59 | 70.00 | 76.00 | 78.58 | 51.62 | 64.29 | 64.00 | 45.24 |
| Abundance % | ||||||||||||
| Plecoptera | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.27 | 0.04 | 0.00 | 1.07 | 2.43 | 6.04 | 10.40 |
| Trichoptera | 0.01 | 0.03 | 0.24 | 0.00 | 0.16 | 0.39 | 11.16 | 1.16 | 16.28 | 3.71 | 1.36 | 1.09 |
| Ephemeroptera | 1.08 | 2.91 | 0.16 | 0.46 | 13.50 | 32.90 | 17.55 | 18.05 | 1.69 | 22.40 | 1.36 | 4.37 |
| Coleoptera | 0.06 | 0.03 | 0.01 | 0.00 | 0.12 | 0.68 | 2.16 | 0.54 | 0.86 | 2.10 | 3.05 | 2.86 |
| Diptera | 57.99 | 71.06 | 68.83 | 26.59 | 35.18 | 39.67 | 29.89 | 30.16 | 47.50 | 57.22 | 30.77 | 19.19 |
| All taxa | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 10.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
Taxonomic richness (the total number of observed taxa) was calculated for the whole assemblage and for the five most represented aquatic insect orders (Plecoptera, Trichoptera, Ephemeroptera, Coleoptera and Diptera) for each faunal entity.
On qualitative data, the Jackknife richness estimator (JACK2) was calculated [26]. The non-parametric Jackknife is known to standardize samples, reducing biases due to sample size dependency [27,28]. Therefore, it was used to compare the richnesses of a cumulative unequal number of samples in our faunal entities. Jackknife estimates were generated for each unit using EstimateS (Version 7, R.K. Colwell, http://purl.oclc.org/estimates); these estimates derived from 1000 random permutations.
Taxonomic richness associated with each habitat type in the catchment represents the gamma diversity (γ diversity), and the richness of a defined faunal entity represents the alpha diversity (α diversity). To measure the contribution of each faunal entity to the catchment richness (i.e., γ diversity), the turnover of taxa was expressed by beta diversity (β diversity), defined as the distinctness of taxa composition between faunal entities. The distinctness was expressed as:
Abundance, Shannon's diversity index (H), and Shannon's equitability (Eq) were also calculated to examine the structure of each faunal entity.
3 Results
3.1 Taxonomic richness and spatio-temporal units at the scale of the catchment
170,157 individuals out of 102 taxa were identified in the 134 samples collected (Appendix A).
Between-class analysis indicates that the criteria ‘station’ explains 50.17% of the total inertia compared with 11.55% for the criteria ‘date’. Inertia values were significant (, number of random matching = 1000). The F1 axis in C.A. illustrated a pollution gradient. Taxa highly tolerant to pollution and extremely resistant to heavy organic loads including Oligochaeta, Chironomini and Helobdella stagnalis (at the negative side of the F1-axis in Fig. 2I) opposed to taxa more sensitive to organic pollution and colonizing oligotrophic habitats such as Eulectra, Sericostoma, Atrichops crassipes (at the positive side of the F1-axis). Taxa able to resist to temporary flows (e.g., Potamopyrgus and Ancylus fluviatilis [29]) or commonly occurring in temporary waters (e.g., Notonecta and Hydrometra) highly contributed to the faunal typology along the F2-axis. The cluster analysis realized on the C.A. data divides our 27 station-date combinations in three spatio-temporal units (Fig. 2II). Unit A includes the four Arc stations at the three dates and the La Partie in February (PAFeb), the entire Arc stations are in the same unit, even Arc1, located upstream of the Trets wastewater treatment plant. Unit B corresponds to the station of the intermittent tributaries, AU and AI at the three dates, and PA in April and June. Unit C includes the stations of the perennial tributaries, CA and BA at the three dates.

Scores of the Correspondence Analysis on the F1 × F2 factorial map. I: Distributions of taxa where only the most contributing taxa are shown. II: Ordination plots showing the distribution of the site-date combinations. A, B and C represent the three spatio-temporal units established from a cluster analysis of the C.A. results.
3.2 Habitat typology
From the dendrogram, four habitat types were deduced, with a level of 70% of heterogeneity (Fig. 3). This level of heterogeneity permitted to separate samples into groups representing differences in habitat criteria ecologically distinctive (i.e. sharp abiotic conditions distinctiveness). The 134 samples were dispatched into these four types of habitats, respectively 18, 37, 41, 38 for habitat types 1, 2, 3 and 4. The most correlated variable to the M.C.A. axis is the substrate (6 modalities). Habitat type 1 was dominated by silt and litter; type 2 by boulder; type 3 by cobble-pebble and roots; and type 4 by sand.

Dendrogram obtained by cluster analysis of the Multiple Correspondence Analysis results (cumulative variance of the first two axes 32%; Euclidean distances were used and the linkage was done following the average linkage method (UPGMA)). Four groups are defined at 70% of heterogeneity (arrow); 1 = samples of habitat type 1, 2 = samples of habitat type 2, 3 = samples of habitat type 3, 4 = samples of habitat type 4.
3.3 Richness patterns and faunal entities structure at the catchment scale
3.3.1 Faunal entities structure
Insects were highly dominated by Diptera with abundance values reaching 71.1% of the total abundance; the highest percentages were observed for unit A (Table 2). The dominance of Diptera was mainly due to Chironomidae (Fig. 4), with differences in composition among the units. Unit A was mainly dominated by Chironomini, unit C dominated by Orthocladiinae, when unit B seemed to have the most equally balanced composition. In unit B, Ephemeroptera was the second relatively abundant insect order after Diptera, with abundances reaching 32.90% of the total abundance in habitat type 2. Trichoptera and Plecoptera had higher relative abundance in unit C than in unit A. They accounted for 17.4% of the total abundance of habitat type 1 and 11.5% of the total abundance of the fourth habitat type of group C. Shannon's diversity index (H) and Shannon's equitability (Eq) showed conspicuous differences between unit A and the other two units (B and C). The lowest values of H and Eq were observed for unit A, with values not exceeding 2.61 for H and 0.53 for Eq.
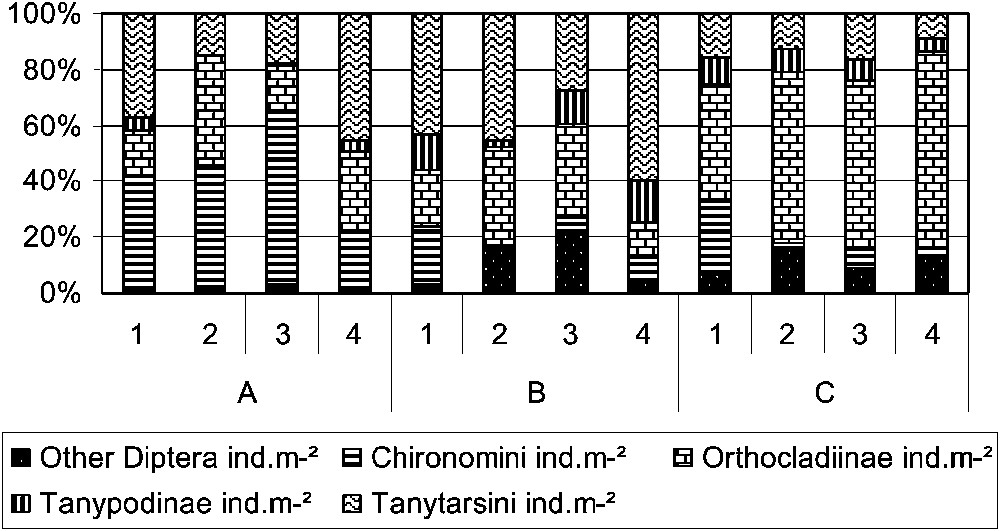
Relative abundances of Diptera taxa in the 12 faunal entities. This graphic shows the main Chironomidae subfamilies and tribes and the other Diptera.
3.3.2 Alpha, beta and gamma diversity
At the catchment scale, gamma diversities values were 62, 70, 75 and 84 for habitat types 1, 2, 3 and 4, respectively.
Based on Jackknife analysis, taxa accumulation curves revealed an asymptote for the twelve faunal entities after an accumulation number of 10 samples, except for B1, C1 and C4 (Fig. 5). Curve profiles showed that taxa richness did not exceed 40 taxa for unit A in the four habitat types, while it reached 70 taxa for unit B in habitat type 4, and almost 50 for unit C in habitat type 3.
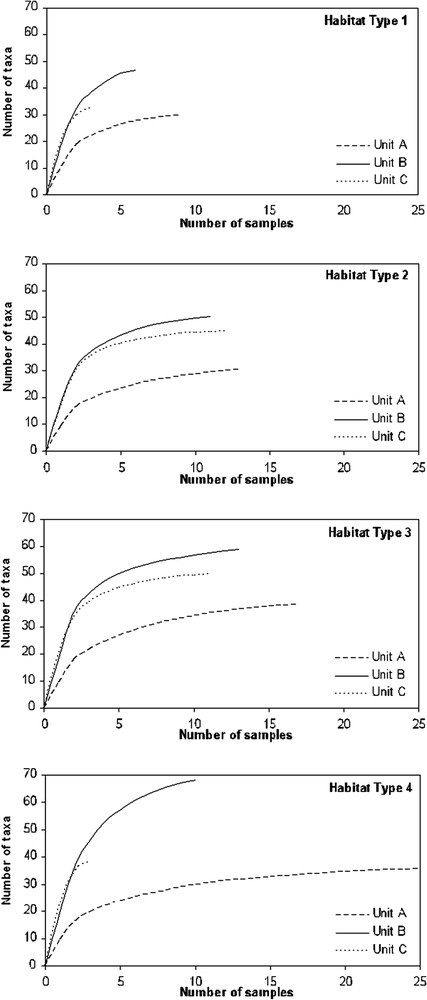
Taxonomic richness estimations of the 12 faunal entities based on a Jackknife analysis. The four graphs represent the four habitat types and in each graph curves of richness estimation of the three spatio-temporal units are drawn (units A, B and C).
Alpha diversity values calculated for each of the 12 faunal entities showed that unit A has the lowest values in each of the four habitat types. Unit B included the highest values of alpha diversity, between 45 and 66, while unit C was in an intermediate position (Table 2). The lowest distinctness values were observed for habitat types of the spatio-temporal unit A (from 41.67 to 49.34), while they ranged from 70.00 to 78.58 for B and from 45.24 to 64.29 for C. Plecoptera are absent from the four faunal entities of the spatio-temporal unit A (Table 2). Faunal entities of this spatio-temporal unit enclosed less than half of the taxa recorded at the catchment scale. Spatio-temporal unit B is characterized by the highest values of distinctness, with two 100 values for the Ephemeroptera (in habitat types 2 and 4) and one for the Coleoptera (in habitat type 4), which means that all the Ephemeroptera and Coleoptera taxa recorded for these habitat types in this catchment are represented in unit B. Spatio-temporal unit C had distinctness values that tended to range between the values obtained in groups A and B, together with values of 100 in the case of Ephemeroptera present in habitat type 3 and the Plecoptera in habitat type 4. Spatio-temporal units B and C harboured together all the Ephemeroptera taxa recorded in habitat types 2, 3 and 4.
4 Discussion
4.1 Patterns of taxa richness
Nowadays, few Mediterranean streams remain undisturbed by anthropogenic activities. Methods of water quality assessment using benthos point out sharp faunal changes due to human activities [12,14,30]. The Arc stream catchment offers a picture of the observed or expected changes in the majority of the European stream ecosystems under Mediterranean climate. Our results show that the four Arc stations are grouped in the same spatio-temporal unit without distinction, while the other five tributary stations were distributed in two units, characterized mainly by their hydrological regime (intermittent versus perennial). The faunal assemblages of station Arc1, upstream of the wastewater processing plant, did not reflect a better ecological status there than at the three Arc stations downstream of the plant. Nor did station Arc4, located at 8.3 km downstream of the effluent, reflect any biological recovery. Those results suggest unexpected stress affecting station Arc1, probably due to diffuse sources of pollution of agricultural origin.
Unit A (Arc stream and PA February) enclosed less than half of the taxa recorded in this part of the catchment, while unit B (intermittent tributaries) supported greater α diversity values than unit C (perennial tributaries). The distinctness of the values improved the position of the intermittent tributaries as the freshwater biota that supported the highest taxa richness in each one of the four habitat types.
Multiple studies pointed out lower taxonomic richness values in intermittent streams than in those with perennial flow regimes [31–33]. This difference was attributed to the hypotheses that favourable environmental conditions (in perennial streams) harbour large numbers of taxa that gradually decrease in richness (in intermittent streams) as they are replaced by more tolerant or opportunistic taxa. In contrast, our study shows that the greatest annual diversity values were observed in the intermittent tributaries in the four habitat types. Distinctness calculations showed that intermittent tributaries harbour 70% of the taxa assessed in this study and H and Eq scores calculated confirm that macroinvertebrate assemblages were more equitable in their composition in the tributary stations in the four defined habitat types. Legier and Talin [18] studied three intermittent and three perennial streams in Provence (southeastern France) in 1973, including one perennial stream and an intermittent one, which are tributaries of the Arc stream. They revealed higher species richness values in the intermittent tributary, with 80 and 43 species in the intermittent and the perennial tributaries, respectively. These studies seem to suggest that the taxonomic richness is greater in intermittent Mediterranean streams than in streams with a perennial flow regime, and that this feature may contribute importantly to sustaining catchments biodiversity.
It may be possible to explain the high taxonomic richness values obtained in the intermittent tributaries in comparison with perennial tributaries in terms of intermediate disturbance hypothesis [34,35], as low taxonomic richness occurs under low levels of disturbance, where highly competitive taxa monopolize the resources. The tributaries CA and BA sustain stable habitats as they have perennial flow, while the AI, AU and the PA have intermittent flow regimes involving a series of different abiotic conditions and thereby a succession of different benthic communities. Intermittent streams generally show wide variations in their physical and chemical parameters, which are much greater than those occurring in most perennial streams. Authors studying the fauna inhabiting intermittent streams [36,37] reported that they belong to three main groups. The first group consists of perennial stream forms not particularly well adapted to life in intermittent streams, the second group consists of taxa occurring in both lotic and lentic waters, and the third group consists of species highly adapted and often restricted to intermittent waters. The benthic macroinvertebrate fauna collected in this study confirm these conclusions. Taxa adapted to intermittent waters as some genera of Coleoptera (Gyrinus, Hydraena, Coelostoma, Ochthebius) and some Hemiptera (Corixidae, Hydrometra, Nepa, Notonecta) were found only in the intermittent tributaries, while taxa like Leuctridae, Capnia and Valvata found previously in the hyporheic zone [38,39] and able to avoid unfavourable conditions, are found in intermittent and perennial tributaries. Intermittent tributaries, where drought is annual evidence, seem to harbour macroinvertebrate communities that are well adapted to temporary flows. This succession of flow and drought periods makes the intermittent tributaries suitable habitats for various macroinvertebrates unable to coexist under stable conditions in perennial streams.
4.2 Tributaries and taxonomic richness: implication for conservation
The severe deterioration of water quality and habitat fragmentation of natural habitats occurring in Europe, especially in the Mediterranean coastal areas, constitute an increasingly serious threat for faunal biodiversity in streams [12,14,40]. This situation is worsened by the fact that insufficient knowledge is available about the biological potential of these streams. The lack of reference sites for these systems makes it very difficult to determine the magnitude of anthropogenic impacts. Small streams, especially the intermittent tributaries of the Mediterranean streams, are among the least thoroughly documented aquatic habitats, although they hold considerable potential for sustaining taxonomic richness and as refuges for invertebrates. These intermittent tributaries preserve a pool of potential colonists for biotopes of the main stem with similar characteristics. Boulton and Suter [32] suggested that typical perennial streams fauna may reside and often successfully reproduce in intermittent systems. This is consistent with the idea that the intermittent tributaries may contribute to the conservation of the pool of taxa present in the catchment.
In the present study, tributaries were found to harbour twice the benthic taxonomic richness observed in the main stem. The highest level of taxonomic richness was observed in tributaries with intermittent flow regimes. As a result of their intermittent flow and low discharge, these tributaries are also highly vulnerable to pollution of various kinds resulting from human activities. Conserving these biotopes is an important aspect of catchment management, as the dispersion of species, which is facilitated by the natural connectivity of these various biotopes, may lead to some species being reintroduced into the main stem. The recruitment potential by downstream/upstream displacement [41] is an important factor contributing to the recovery of the benthic invertebrate richness in disturbed parts of the main channel, and ensuring that the ecological integrity of these tributaries is maintained seems to be a vital means of sustaining the biodiversity in this and other Mediterranean catchments. Finally, the contribution of tributaries to biodiversity pleads for a catchment-scale conservation of freshwater invertebrates [42].
Acknowledgements
This project is financially supported by ‘Conseil général des Bouches-du-Rhône, Service de l'eau et des milieux aquatiques’. We wish to thank Bénédicte Vautrin and Valentine Cartier, who helped in fieldwork and sample processing.
Appendix A Macroinvertebrate taxa list with their presence in the 12 faunal entities; + for present and − for absent
| 1 | 2 | 3 | 4 | ||||||||||
| A | B | C | A | B | C | A | B | C | A | B | C | ||
| Amphipoda | Gammarus | + | + | + | + | + | + | + | + | + | + | + | + |
| Arhynchobdellida | Erpobdella | − | − | − | − | − | − | − | − | − | + | − | − |
| Erpobdellidae | − | − | − | − | + | − | − | + | − | − | + | − | |
| Basommatophora | Ancylus fluviatilis | + | + | − | + | + | + | + | + | − | + | + | − |
| Physa | + | + | − | + | + | − | + | + | − | + | + | + | |
| Stagnicola | + | − | − | − | − | − | + | − | − | + | − | − | |
| Coleoptera | Brychius | + | − | − | − | − | − | − | − | − | − | − | − |
| Coelostoma | − | − | − | − | + | − | − | − | − | − | + | − | |
| Donacia | − | − | − | − | − | − | − | − | − | − | + | − | |
| Dryops | − | + | − | + | − | + | + | − | + | − | + | − | |
| Dytiscidae | − | + | − | − | + | − | − | − | − | − | + | + | |
| Elmis | + | + | − | + | + | + | − | + | + | − | + | + | |
| Esolus | + | − | + | + | + | + | + | + | + | − | + | + | |
| Gyrinus | − | + | − | − | + | − | − | − | − | − | + | − | |
| Haliplus | + | + | − | − | − | − | − | − | − | − | − | − | |
| Coleoptera | Helodidae | − | − | − | − | − | + | − | + | + | − | + | + |
| Hydraena | − | − | − | − | − | − | − | − | − | − | + | − | |
| Hydroporinae | + | + | − | − | + | − | − | − | − | − | + | − | |
| Limnius | − | + | + | + | − | + | + | + | + | − | + | + | |
| Ochthebius | − | − | − | − | − | − | − | + | − | − | + | − | |
| Orectochilus | − | − | − | − | − | + | − | − | − | − | − | − | |
| Oulimnius | − | − | − | + | − | − | + | + | − | + | + | − | |
| Riolus | − | − | + | − | + | + | − | + | + | − | + | + | |
| Diptera | Atherix | − | − | − | − | − | − | + | − | − | − | − | − |
| Atrichops crassipes | − | + | + | − | − | + | + | − | + | − | + | + | |
| Ceratopogonidae | + | + | + | + | + | + | + | + | + | + | + | + | |
| Chironomini | + | + | + | + | + | + | + | + | + | + | + | + | |
| Clinocerinae | − | − | + | − | − | + | − | + | + | − | + | + | |
| Culicidae | − | − | − | − | − | − | + | − | − | + | − | − | |
| Dicranota | − | − | − | − | + | − | − | + | − | − | + | − | |
| Dixa | − | − | − | − | − | − | − | + | + | − | + | − | |
| Empididae | − | − | − | − | − | − | − | − | + | − | + | + | |
| Hemerodromiinae | − | + | + | + | + | + | − | + | + | − | − | + | |
| Limoniini | − | + | + | − | − | + | + | − | + | − | − | + | |
| Orthocladiinae | + | + | + | + | + | + | + | + | + | + | + | + | |
| Pediciini | − | − | − | + | − | − | + | − | − | + | − | − | |
| Pericoma | − | − | − | − | − | − | − | + | − | − | − | − | |
| Psychodidae | − | + | − | + | + | + | − | + | + | − | + | − | |
| Ptychopteridae | + | − | − | − | − | − | − | − | − | + | − | − | |
| Rhagionidae | − | − | − | − | + | + | − | − | − | − | − | − | |
| Simuliidae | + | + | − | + | + | + | + | + | + | + | − | + | |
| Stratiomyidae | − | + | − | − | + | − | − | + | − | − | − | − | |
| Tabanidae | − | − | − | − | − | + | + | + | − | − | + | + | |
| Tanypodinae | + | + | + | + | + | + | + | + | + | + | + | + | |
| Tanytarsini | + | + | + | + | + | + | + | + | + | + | + | + | |
| Tipulidae | + | + | − | − | + | − | + | + | − | + | + | − | |
| Ephemeroptera | Baetis rhodani | + | − | − | + | + | + | + | + | + | + | + | + |
| Caenis | + | + | + | + | + | − | + | + | + | + | + | + | |
| Centroptilum | − | − | + | − | + | + | − | + | + | − | + | + | |
| Cloeon | − | + | − | − | − | − | − | − | − | − | + | − | |
| Ecdyonurus | − | − | − | − | + | − | − | − | − | − | − | − | |
| Ephemera | − | − | + | − | + | + | − | + | + | − | + | + | |
| Ephemerella | − | + | − | + | + | + | − | + | + | − | + | − | |
| Habrophlebia | + | + | + | + | + | + | + | + | + | + | + | + | |
| Paraleptophlebia | − | − | + | − | + | − | − | − | + | − | − | + | |
| Hemiptera | Corixidae | − | + | − | − | − | − | − | − | − | − | − | − |
| Gerris | − | − | − | − | − | + | − | − | + | − | + | − | |
| Hydrometra | − | − | − | − | + | − | − | + | − | − | + | − | |
| Nepa | − | − | − | − | − | − | − | + | − | − | + | − | |
| Notonecta | − | + | − | − | − | − | − | − | − | − | + | − | |
| Veliidae | − | − | − | − | + | − | − | + | − | − | + | − | |
| Heterostropha | Valvata | − | − | − | − | + | − | − | − | − | − | + | − |
| Hygrophila | Ferrissia | − | − | − | − | − | − | − | − | − | + | − | − |
| Lymnaeidae | + | + | + | + | + | + | − | + | + | + | + | + | |
| Planorbidae | − | − | − | − | − | − | − | + | − | − | − | − | |
| Isopoda | Asellus | + | + | − | + | − | − | + | − | − | + | + | + |
| Megaloptera | Sialis | − | − | − | − | − | − | − | − | − | + | − | − |
| Neotaenioglossa | Bithynia | − | − | − | − | − | − | − | − | − | + | − | − |
| Odonata | Aeshnidae | − | − | + | − | − | + | − | + | + | − | − | − |
| Calopteryx | − | + | + | − | − | − | − | + | + | − | + | − | |
| Coenagrionidae | − | + | − | − | − | − | − | + | − | − | + | − | |
| Cordulegaster | − | − | − | − | + | + | − | + | + | − | + | − | |
| Corduliidae | − | − | − | − | − | − | − | − | − | − | + | − | |
| Gomphus | − | − | − | − | − | + | − | − | − | − | − | − | |
| Lestidae | − | + | − | − | − | − | − | − | − | − | + | − | |
| Libellulidae | − | − | − | − | − | − | − | − | − | + | + | − | |
| Onychogomphus | − | − | + | − | + | + | + | + | + | + | + | + | |
| Oligochaeta | Oligochaeta | + | + | + | + | + | + | + | + | + | + | + | + |
| Plecoptera | Capnia | − | − | − | − | + | − | − | − | − | − | − | − |
| Euleuctra | − | − | + | − | − | + | − | − | + | − | − | + | |
| Isoperla | − | − | − | − | + | − | − | + | − | − | − | − | |
| Leuctridae (other) | − | + | − | − | + | − | − | − | + | − | − | + | |
| Nemoura | − | − | − | − | + | − | − | − | + | − | − | + | |
| Rhynchobdellida | Helobdella stagnalis | + | + | − | + | − | − | + | − | − | + | + | − |
| Glossiphoniidae (other) | + | + | − | − | − | − | − | − | − | − | − | − | |
| Sorbeoconcha | Potamopyrgus | + | + | + | + | + | + | + | + | + | + | + | + |
| Trichoptera | Agraylea | − | − | − | − | − | − | + | + | − | − | − | − |
| Goeridae | − | − | − | − | − | − | − | + | − | − | − | − | |
| Hydropsyche | − | + | + | + | + | + | + | + | + | + | + | + | |
| Hydroptila | − | − | + | − | + | + | + | − | + | + | − | − | |
| Limnephilidae | − | + | − | − | + | − | − | + | + | − | + | − | |
| Lype | − | − | + | − | − | − | − | + | + | − | + | − | |
| Mystacides | − | + | + | − | − | + | − | − | − | − | − | − | |
| Oecetis | − | − | − | − | − | − | − | − | + | + | − | − | |
| Polycentropus | − | + | − | − | − | − | + | + | + | + | + | − | |
| Rhyacophila | + | − | − | + | + | + | + | + | − | − | + | − | |
| Sericostoma | − | + | + | − | − | + | − | + | + | − | + | + | |
| Tinodes | − | − | − | + | − | + | − | + | + | − | + | + | |
| Wormaldia | − | − | − | − | + | + | + | + | − | − | + | − | |
| Tricladia | Dugesia | + | + | + | − | − | + | + | + | + | + | + | − |
| Veneroida | Pisidium | − | + | + | − | + | + | − | + | + | − | + | + |
| Sphaerium | + | − | − | + | − | − | + | − | − | + | − | − |


