Version française abrégée
Le cadmium (Cd) est un métal lourd, n'ayant aucun rôle biologique reconnu, provoque des effets néfastes chez tous les êtres vivants, en particulier chez les végétaux. Cet élément peut entraîner une perturbation dans certains processus physiologiques et métaboliques. Dans ce travail, nous avons exploré les effets de l'augmentation du stress cadmique (0, 10, 20, 50 et 100 μM CdC2) sur la croissance, la nutrition et le métabolisme azotés chez des plantules de tabac (Nicotiana tabaccum, Bureley v.).
Les résultats obtenus montrent que l'addition du Cd dans le milieu de culture entraîne une réduction de la croissance des parties aériennes et des racines. Cet effet dépressif du Cd est beaucoup plus prononcé au niveau du système racinaire que des parties aériennes.
Toutefois, la toxicité de Cd2+ est étroitement liée à la capacité des plantes à retenir ce métal dans le système racinaire, afin de limiter son exportation vers le parenchyme foliaire très vulnérable à l'égard de ce polluant. Des travaux antérieurs ont montré que chez les plantes herbacées, la quasi-totalité du Cd absorbé est retenue au niveau des racines. Pour la première fois, nous avons montré que chez le tabac et pour tous les traitements, le Cd est essentiellement accumulé au niveau des feuilles. La grande partie du Cd absorbé (97%) est transportée vers les parties aériennes et reste localisée au niveau des limbes foliaires. A ce niveau, le Cd diminue les teneurs en pigments photosynthétiques, notamment celles des chlorophylles b. De tels effets peuvent s'expliquer en prenant en considération que l'application du Cd peut conduire à une déficience en fer et en magnésium au niveau des feuilles. Ces éléments nutritifs sont essentiels à la nutrition de la plante et font partie des métalloprotéines impliquées dans la biosynthèse des caroténoïdes et de la chlorophylle.
Au niveau nutritionnel, l'augmentation de la dose de Cd dans le milieu entraîne une diminution de la teneur en eau dans tous les organes de la plante. Ces perturbations des paramètres hydriques peuvent être interprétées comme le résultat d'une interaction entre le métal et la régulation stomatique et/ou résultant d'une baisse de la conductivité hydraulique au niveau de la tige suite aux effets du Cd sur les tissus conducteurs. De même, le traitement cadmique est accompagné d'une nette diminution de la teneur en nitrate dans les différents tissus de la plante, relativement aux témoins. Cette baisse des teneurs en nitrate est plus prononcée dans les tissus foliaires où s'effectue une accumulation considérable du Cd par rapport aux tissus racinaires. La baisse des teneurs en nitrate sous l'effet du Cd peut être due à une restriction de l'absorption du nitrate. Plusieurs hypothèses ont été avancées pour expliquer la diminution de la capacité d'absorption racinaire du nitrate par le Cd. Ceci peut résulter d'une altération de la perméabilité membranaire par le Cd, probablement due à une modification des constituants lipidiques majeurs du plasmalemme. De même, on ne peut exclure l'effet de Cd sur l'expression et la synthèse des protéines de transport membranaire.
La restriction de l'alimentation en nitrate peut éventuellement avoir des conséquences sur les voies métaboliques dépendantes de cet élément, en particulier les processus de réduction et d'assimilation de l'azote minéral.
Nous avons constaté que l'exposition des plantes pendant 7 jours aux différentes doses de Cd, entraîne une inhibition des activités de la nitrate réductase (NR) et de la nitrite réductase (NiR), de façon plus prononcée dans les racines que dans les feuilles. Il semble que la corrélation entre ces deux enzymes de la réduction du nitrate est nécessaire pour éviter toute accumulation excessive des ions nitrites, toxiques pour la cellule. Toutefois, la NR s'est avérée plus sensible au Cd que la NiR. Cette différence de sensibilité au Cd entre les deux enzymes serait probablement liée à leur différente localisation au niveau cellulaire : la NiR est localisée au niveau des chloroplastes et des proplastes racinaires. Ces organites cellulaires sont à membrane double et de ce fait, l'enzyme n'est pas en contact direct avec le Cd. Il a été prouvé également que la NiR est une protéine plus stable que la NR.
En aval, l'ammonium, produit par la réduction du nitrate, est assimilé par la glutamine synthétase (GS) pour produire la glutamine. L'addition du Cd dans le milieu de culture, provoque une nette inhibition de l'activité GS jusqu'à la dose 50 μM, cet effet est plus marqué dans les racines que dans les feuilles. Il serait possible que cette inhibition de l'activité GS résulte d'un effet direct du Cd sur la protéine enzymatique, provoquant ainsi son inactivation partielle. L'inhibition directe de l'activité GS par les ions Cd2+ traduit probablement une altération de l'activité catalytique de l'enzyme. Cette altération résulte d'une interaction du Cd avec les résidus thiols qui sont indispensables pour l'activité catalytique de l'enzyme.
Contrairement à la diminution de la synthèse de la glutamine par l'activité GS, le traitement cadmique stimule la production du glutamate grâce à l'activité aminatrice de la glutamate déshydrogénase (NADH-GDH), aussi bien des tissus foliaires que racinaires. Parallèlement, nous avons enregistré une augmentation des niveaux endogènes des ions ammonium dans tous les organes des plantes traitées. Cette augmentation progressive des teneurs en ammonium sous l'effet du Cd est probablement le résultat de la dégradation des protéines, du fait que le stress cadmique provoque une stimulation de l'activité des protéases au niveau des feuilles et des racines. Dans ces conditions, la fonction aminatrice de la GDH joue un rôle dans la détoxification des ions ammonium, excessivement cytotoxiques.
En conclusion, malgré, les niveaux d'accumulation du Cd nettement plus élevés dans les tissus foliaires, les effets inhibiteurs de ce métal sur l'activité des enzymes du métabolisme azoté sont plus accentués au niveau des racines. Il semble que les feuilles de tabac sont capables de gérer le Cd absorbé de façon à protéger l'activité métabolique cellulaire. L'accumulation préférentielle du Cd absorbé au niveau de la partie aérienne, suggère que cette variété de tabac peut être recommandée pour la phytorémédiation des sols contaminés par le Cd. Des études plus poussées, pourront apporter des explications quant aux modalités de compartimentation et de neutralisation des ions Cd+2 au niveau des cellules foliaires.
1 Introduction
Heavy metals are serious environmental pollutants and their toxicity is a problem of increasing consequences for ecological, evolutionary, nutritional and environmental reasons [1,2]. Cadmium (Cd) is one of the heavy metals which is the most toxic when dispersed in the environment. The origins of Cd pollution are several. Cadmium could outcome through phosphate fertilizers, sewage sludges and atmospheric repercussions [3].
Tobacco too, can be considered as another origin of heavy metal contamination of the environment by Cd: One cigarette contains at least 16 to 24 μg of cadmium [3]. This high Cd contents is a major source of toxicity and human health problems in that contamination is caused by inhalation. For instance, 90% of Cd is absorbed by the breathing system [1]. Thus the risk of toxicity by Cd on human beings is manifold.
Cadmium had also many harmful effects on plants. With raised amounts, Cd caused a reduction of tobacco plant growth [4]. This effect on growth could result from the decrease in nitrate uptake and reduction [5]. Nitrate reductase (NR) and nitrite reductase (NiR) require nitrate for their induction [6]. Nitrate uptake and transport appear to be sensitive to Cd stress, and this may have severe consequences for nitrate reduction in plants [7].
Ammonium originated from direct absorption, NR/NiR activities, photorespiration, dinitrogen fixation or protein catabolism, is assimilated by the glutamine synthetase (GS) and the glutamate synthase (Fd-GOGAT and NADH-GOGAT, EC 1.4.7.1) [8]. Under special conditions, glutamate dehydrogenase (GDH) is also able to generate glutamate [9].
In order to assess the Cd stress-induced effects on growth and nitrogen metabolism steps, tobacco seedlings were exposed to increasing Cd concentrations. The responses obtained of nitrogen-assimilating enzymes are discussed in relation to the Cd accumulation and changes in metabolite contents in plant leaves and roots.
2 Materials and methods
2.1 Plant material and growth conditions
Seeds of tobacco (Nicotiana tabaccum, Bureley V. Fb9) given by “El Agricola” (Italy), were germinated on moistened filter paper at 25 °C in the dark. The seedlings obtained were transferred to continuously aerated nutrient solutions containing KNO3 8 mM, Ca(NO3)2 2 mM, KH2PO4 1 mM, MgSO4 1 mM, Fe-K-EDTA 32.9 μM, and micronutrients: H3BO4 30 μM, MnSO4 5 μM, CuSO4 1 μM, ZnSO4 1 μM, (NH4)6Mo7O24 1 μM. Plants were grown in a growth chamber: 26 °C/70% relative humidity during the light period and 20 °C/90% relative humidity during the dark period; photoperiod: 16 h daily with a light irradiance of 150 μmol m−2 s−2 at the plant canopy. Plants were grown for 30 days in control medium, and then cadmium treatments (10, 20, 50 and 100 μM CdCl2) were applied during 7 days.
2.2 Nitrate contents
Nitrate ions were extracted from dry matter with 0.5 N H2SO4 at room temperature for 48 h. Nitrate was colorimetrically determined on an automatic analyzer following diazotation of the nitrite obtained by reduction of on a cadmium column.
2.3 Ammonium contents
Ammonium was extracted from plant material at 4 °C with 0.3 mM H2SO4 and 0.5% (w/v) polyclar AT. Ammonium content was quantified according to the reaction of Berthelot modified by Weatherburn [10].
2.4 Cadmium content
Cadmium content in various plant tissues was analyzed by digestion of dried samples with an acid mixture (HNO3/HClO4, 4/1 v/v). Cadmium concentrations were determined by atomic absorption spectrophotometry (Perkin-Elmer, Analyst 300).
2.5 Protein content
Soluble protein content was quantified using Coomassie Brilliant blue [11] with bovine serum albumin as a protein standard.
2.6 Chlorophyll determination
Chlorophyll was determined by the method of Arnon [12]. The absorbance of each sample was read at 460, 645 and 663 nm, after centrifugation.
2.7 Enzyme assays
2.7.1 Nitrate reductase
Frozen plant material (PMF) was homogenized in a chilled mortar and pestle with 100 mM potassium phosphate buffer (pH 7.4) containing 7.5 mM cystein, 1 mM EDTA and 1.5% (w/v) casein. The homogenate was centrifuged at 30,000 g for 15 min at 4 °C. Nitrate reductase activity (NRA) was determined according to the method described by Robin (1979) [13]. The extract of 0.1 mL was incubated in a reaction mixture containing 0.5 mL of 0.1 M potassium phosphate buffer (pH 7.4), 0.1 mL of 0.15 mM NADH, and 0.1 mL of 0.1 M KNO3 at 30 °C for 30 min. The extract was incubated with MgCl2 10 mM (for actual NRA determination) or with excess of 15 mM EDTA (for maximum NRA determination). The reaction was stopped by 0.2 mL of 1 M zinc acetate. Nitrite ions were assayed after diazotation with 1 mL of 5.8 mM sulfanilamide, 1.5 N HCl, and 1 mL of 0.8 mM N-naphthyl-ethylene-diamine-dichloride.
2.7.2 Nitrite reductase
Enzyme extracts were prepared as described above for nitrate reductase. Nitrite reductase was assayed by the method of Losada and Paneque [14]. The extract of 0.1 mL was incubated in a solution containing 0.4 mL of 0.1 M potassium phosphate buffer (pH 7.4), 0.1 mL of 15 mM sodium nitrite, 0.2 mL of 5 mM methyl viologen, 0.2 mL of 86.15 mM sodium dithionite in a 190 mM NaHCO3. The reaction was stopped by a violent agitation on vortex. Nitrite ions were assayed as described for NRA assay.
2.7.3 Glutamine synthetase
Frozen samples were homogenized in a cold mortar and pestle with grinding medium containing 25 mM Tris-HCl buffer (pH 7.6), 1 mM MgCl2, 1 mM EDTA, 14 mM β-mercaptoethanol and 1% (w/v) polyvinylpyrrolidone (PVP). The homogenate was centrifuged at 25,000 g for 30 min at 4 °C. GS activity was determined using hydroxylamine as substrate, and the formation of γ-glutamylhydroxamate (γ-GHM) was quantified with acidified ferric chloride [15].
2.7.4 Glutamate dehydrogenase
GDH extraction was performed according to the method described by Magalhaes and Huber (1991) [16]. Frozen samples were homogenized in a cold mortar and pestle with 100 mM Tris-HCl (pH 7.5), 14 mM β-mercaptoethanol, and 1% (w/v) PVP. The extract was centrifuged at 12,000 g for 15 min at 4 °C. GDH activity was determined by following the absorbance changes at 340 nm [17].
2.8 Protease
Protease activity was measured by the method of Weckenmann and Martin [18], using azocasein as substrate. Absorbance of the released-azo-dye was measured at 340 nm and one unit of activity was defined as the activity producing an increase of 0.01 unit of absorbance during 1 h incubation.
2.9 Statistical analysis
The data are presented in the figures and in the tables as the average of at least six replicates per treatment and means ± confidence limits at level. Each experiment was conducted in duplicate.
3 Results
3.1 Growth response to cadmium
Cadmium treatment leads to a progressive decrease in leaf surface area (Fig. 1A). This reduction reached 63% at 100 μM Cd treatment. The leaf and root DW production was gradually decreased with increasing cadmium concentration in the nutrient medium (Fig. 1B).

Effects of Cd treatments (0, 10, 20, 50 100 μM) for 7 days on (A) leaf area, (B) dry weight (DW) production, (C) water contents, (D) Soluble protein contents, and (E) Chl a, Chl b and carotenoïds contents. Data are means of six replicates ± CL at 0.05 levels.
At low Cd treatment (10 μM), the root growth was more affected than leaves (Fig. 1B). At high Cd treatments (100 μM), the reduction of DW production was 70 and 60% with reference to controls in the roots and leaves, respectively (Fig. 1B).
The growth inhibition of tobacco seedlings was accompanied by a decrease in water contents (Fig. 1C). The leaf hydration was significantly reduced at 100 μM Cd; for witch water content was decreased by about 55% in the leaves and 45% in the roots (Fig. 1C).
The decrease of DW production and surface area in leaves was associated with a reduction of chlorophyll content (Chl a and Chl b) (Fig. 1E). Soluble protein contents were gradually decreased until the 50 μM Cd treatment when they decreased by about 30% in the leaves and 35% in roots (Fig. 1D). The highest Cd stress (100 μM) resulted in a lesser decrease in soluble protein contents in the leaves (25%) and roots (20%).
3.2 Cd, and contents
Cadmium ions were accumulated at higher levels in the leaves than in roots. At 10 μM Cd, the leaves accumulated more than 97% of total absorbed cadmium by the plant (Fig. 2A).
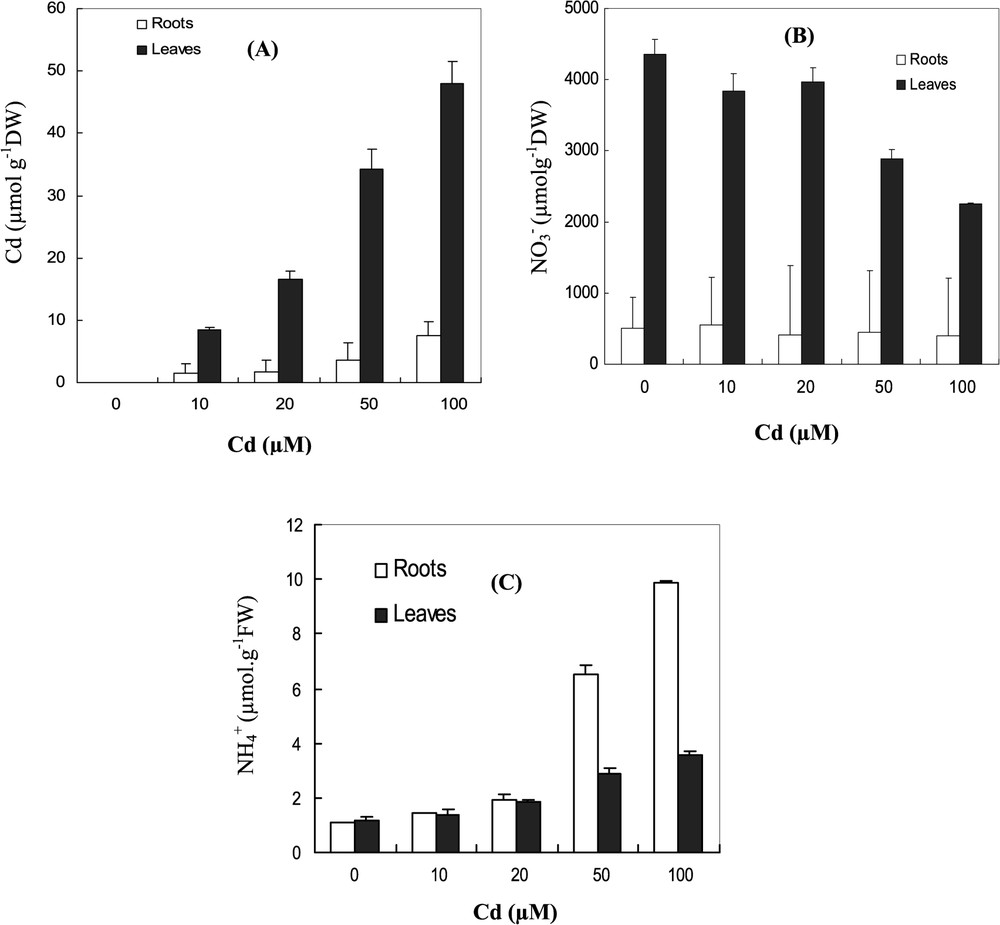
Changes in contents (μmol/g DW) of (A) Cd, (B) , and (C) in leaves and roots under Cd treatments (0, 10, 20, 50, 100 μM) for 7 days. Data are means of six replicates ± CL at 0.05 levels.
In control plants, the leaf nitrate content was 8-times more important than root nitrate contents (Fig. 2B). Under increasing concentration of Cd, contents were greatly decreased in both leaves and roots. At 100 μM Cd, the decrease of content was more severe in the leaves; it reached 50% and only 20% in roots, with respect to control. Ammonium contents in the leaves and roots significantly increased at 50 and 100 μM Cd treatments. At 100 μM Cd, ammonium contents amounted by 90 and 65% relative to control in the leaves and roots, respectively (Fig. 2C).
3.3 Effects of CdCl2 on the nitrogen-assimilating enzymes
3.3.1 Nitrate reductase activity
In the control tobacco seedlings, nitrate reductase (NR) activity was higher in leaves than in roots (Fig. 3A). Under the different Cd treatments, leaf NR activity was more important than root NR activity. At 100 μM Cd, leaf NR activity was 24 times more important than root NR activity (Fig. 3A). Cd stress provoked a decrease in leaf and root NR activities. The NR reduction was more important in roots (80%) than in leaves (60%).
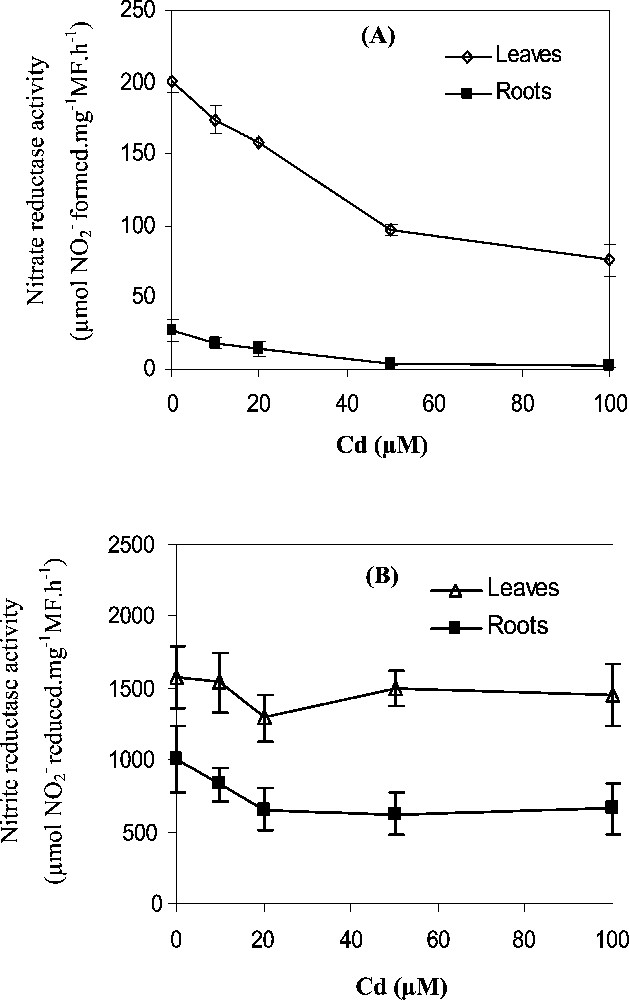
Effects of Cd treatments (0, 10, 20, 50, 100 μM) for 7 days on (A) Nitrate reductase activity (μmol formed. g−1 FW h−1) and (B) Nitrite reductase activity (μmol reduced. g−1 FW h−1) in the leaves and roots. Data are means of six replicates ± CL at 0.05 levels, CL are not shown when they are smaller than the symbol.
3.3.2 Nitrite reductase activity
The reduction of nitrite ions formed by NR activity is carried out primarily in the leaves (Fig. 3B). The NiR activity was greater than NR activity in the leaves (8-fold) and in the roots (40-fold) (Fig. 3A, B). The addition of Cd in the culture medium caused a slight decrease of NiR activity in the leaves which was less than 10% at 100 μM treatment. In the roots, NiR activity was more affected; it was decreased by about 35% at 100 μM Cd treatment.
3.3.3 Glutamine synthetase activity
In the control plants, leaf GS activity was 4 times more important than that of roots (Fig. 4A). The inhibitory effect of Cd on GS activity appeared with the lowest Cd treatment (10 μM) in the roots (20% ) but not in leaves. At 50 μM, GS activity was decreased by about 30% in leaves and by more than 70% in roots with respect to control plants (Fig. 4A). At 100 μM Cd treatment, GS activity increased in the leaves and roots, while it remained at lower levels than controls. The reduction of GS activity was about 20% in the leaves and roots (Fig. 4A).

Effects of Cd treatments (0, 10, 20, 50, 100 μM) for 7 days on (A) GS activity (μmol γ-glutamylhydroxamate (GHM). g−1 FW h−1), (B) NADH-GDH activity (μmol NADH oxidized. g−1 FW h−1) and (C) NAD-GDH activity (μmol NAD reduced. g−1 FW h−1) in the leaves and roots. Data are means of six replicates ± CL at 0.05 levels, CL are not shown when they are smaller than the symbol.
3.3.4 Glutamate dehydrogenase activity
In control plants, GDH aminating activity (NADH-GDH) was about three times more important in the roots than in leaves (Fig. 4B). In both organs, aminating GDH activity was more important than deaminating activity (NAD-GDH) (Fig. 4C). Under Cd treatments, the aminating GDH activity was enhanced in the roots and especially in leaves (Fig. 4B). At 100 μM Cd, the aminating GDH activity was stimulated by 35% in the roots and more than 55% in leaves, with respect to controls (Fig. 4B).
In stressed seedlings, GDH deaminating activity (NAD-GDH) was stimulated in leaves. NAD-GDH activity in leaves was at least two times higher than controls at high Cd treatment. In the roots, Cd stress had an inhibitory effect on the deaminating activity which was decreased by 80% at 100 μM Cd treatment (Fig. 4C).
3.4 Protease activity
Protease activity was enhanced in both roots and leaves by Cd stress (Fig. 5). The root protease activity was about 2 times more important than leaves (Fig. 5). At 100 μM Cd treatment, protease activity increased to become three times higher in the roots and leaves than control plants.

Effects of Cd treatments (0, 10, 20, 50, 100 μM) for 7 days on protease activity (units/g FW/h) in tobacco leaves and roots. Data are means of six replicates ± CL at 0.05 levels, CL are not shown when they are smaller than the symbol.
4 Discussion
Our data show that Cd content in the leaves and roots did not follow the same trends according to Cd exposure in the same variety of tobacco (Bureley), which has been reported by Bovet (2006) [19]. Cadmium was accumulated essentially in leaves: at 100 μM treatment, Cd contents in the leaves were at least five times higher than in roots. This data was in accordance with those described by De Borne et al. (1998) and Lugon-Moulin et al. (2004) [20,21].
Cadmium provoked a considerable reduction in growth of tobacco seedlings (Fig. 1B). This effect was mainly observed in the roots, while leaves were apparently damaged only by the highest Cd concentrations (50 and 100 μM), displaying a decline in leaf surface area (Fig. 1A) and in chlorophyll contents (Fig. 1E). In plant species, cadmium stress led to a leaf yellowing related to chlorophyll breakdown [22]. This decrease could cause in part a photosynthesis and growth reduction [23,24].
Effects of Cd on growth were associated with a decrease in water content in roots and leaves (Fig. 1C). The perturbation of water-relationships is one of the primary effects of Cd toxicity [25,26], and has been interpreted through effects of Cd on stomata functioning [26]. The low water nutrition on Cd-treated plants may impair nutrient uptake.
Ion analysis showed that Cd stress led to a decrease in contents in the roots and leaves (Fig. 2B). It is known that nitrate uptake is mediated by root cell plasmalemme transporters, and is driven by energetic coupling to the transmembrane H+ gradient [27]. Impairment of nitrate transport could thus be a consequence of decreased plasma membrane potential difference due to the inhibition of ATPase: H+ pumps by Cd2+ ions [28]. Cadmium stress may also impair the plasmamembrane integrity by increasing lipid peroxidation [29,30]. Alternatively, it could alter plasma membrane permeability [6] and affect hence nitrate and other nutrient uptake.
The observed decrease in nitrate contents in leaves and roots (Fig. 2B), could affect the subsequent processes involved in nitrate reduction and assimilation. In fact, regulates the NR and NiR expressions [31] and activities [32,33].
The first step in nitrate assimilation is its reduction to nitrite catalysed by NR. In tobacco seedlings, leaf NR activity was 24 times more important than root NR activity (Fig. 3A). In many plants, this elevated nitrate reduction in the leaves relative to the roots related to the higher NR protein contents [34] and a sufficient availability of light and reducing power [35].
After exposing tobacco seedlings to Cd for 7 days, we obtained a significant decrease in NR activity, which was more pronounced in the roots than in leaves (Fig. 3A). This NR activity inhibition was associated with a severe decrease in nitrate content in leaves (Fig. 2B). The nitrate content is closely linked to the nitrate influx [36], which plays a direct role for NR protein production and activation [31]. Thereafter, the Cd-induced inhibition of NR activity in the leaves may result from the low nitrate availability at the enzyme reduction site. Also, direct effects of Cd2+ ions on NR protein are not excluded. In fact, when assayed with control maize (Zea mays L.), in vitro NR activity decreased by addition of Cd in the enzyme incubation medium [37].
The decrease of NR activity in Cd-treated plants could also mirror an increase in the enzyme breakdown induced by toxic oxygen species generated during stress treatment. Indeed free radicals can cause the breakdown of proteins directly by oxidative reaction [38] or indirectly by increasing proteolytic activity [39]. The NiR activity was less affected by Cd stress than the NR activity. For instance, at 100 μM Cd, NiR activity was reduced by only 10% in leaves and 35% in roots, while the reduction of NR activity was 60% in leaves and 90% in roots. The highest NiR activity in the leaves and the roots, insure avoidance of toxic accumulation of nitrite ions [40,41].
With increasing Cd concentration, NiR activity was slightly decreased in the leaves and mainly in the roots (Fig. 3B). These results were in agreement with those of Hernandez et al. (1997) [6]. NiR activity inhibition in tobacco can be considered as a direct consequence of the decrease in NR activity (Fig. 3A) and in contents (Fig. 2B). In fact, it was shown that both NiR and NR were induced by nitrate [31,42].
The ammonium produced by NiR activity is then incorporated into an organic form primarily by the GS enzyme [8,43]. The presence of Cd in the nutrient solution caused a significant decrease in GS activity in leaves and in roots (Fig. 4A). At 100 μM Cd treatment, GS activity increased in both leaves and roots, but did not recover the control value. The GS activity increase in the leaves and in roots was probably related to the induction of the cytosolic GS isoform (GS1) protein and transcripts [5].
Conversely, Cd treatment resulted in an increase in contents in the leaves and in roots (Fig. 2C). The greater increase in ammonium content in the roots coincides with the higher decrease in soluble protein content relative to that obtained in the leaves (Fig. 1D). These results were in accordance with the higher protease activity in the roots than in the leaves (Fig. 5). Cadmium stress may affect the nitrogen enzyme activities by enzyme protein alterations. It was suggested that Cd2+ ions might affect the activity of some enzymes by binding to sulphydryl groups, thus inactivating enzymes [44,45]. The decline of nitrogen enzymes activities upon addition of Cd is likely to be a consequence of a direct interaction between the metal and the SH groups at the enzymes active site.
On the other hand, Cd stress was found to increase the aminating GDH activity in the leaves and roots, even at high Cd concentrations (50 and 100 μM). The high increase of ammonium content may be responsible for aminating GDH activation in both leaves and roots [46]. Aminating GDH activity seems to be involved in the ammonium detoxification under stress conditions [41,47]. Recently, Damianos et al. (2006) [9]. using 15N for in vivo GDH measurement, showed that aminating GDH pathway was actually activated in tobacco leaves by various abiotic stresses.
5 Conclusion
Tobacco plants accumulated Cd mostly in leaves. Leaf Cd content was six times more important than root Cd content. However, tobacco leaves were less affected by Cd stress than roots. The lower sensitivity of leaves to Cd than roots could be related, at least in part, to: (i) a lesser inhibition of nitrate reduction and ammonium assimilation, concomitantly with a high increase in both aminating and deaminating GDH activities under Cd stress; (ii) an ability of leaves to accumulate this metal in non-active forms [48,49]. Tobacco leaves could accumulate Cd in the apoplast, by ionic interactions with carboxyl and/or sulphhydryl groups from components of the cell wall [50]. Part of the metal could be complexed by phytochelatins or other ligands and sequestered in vacuole [51]. The large accumulation of Cd in leaves propose tobacco plants for Cd phytoextraction. Although, more work is needed at the molecular level for further information towards the subcellular accumulation of Cd and its effects on protein and gene expression.
Acknowledgements
Tobacco seeds were kindly given by Mr. Abdeltif Ben Hsin (Direction Agriculture, Régie Nationale des tabacs et des Allumettes, Tunisie). Authors are grateful to Mr. Khaled Jebahy from the “Institut Supérieur de Biologie Appliquée de Médenine” for proofreading the manuscript.


