1 Introduction
The edible yam (Dioscorea cayenensis–D.rotundata complex) has been classified as one of the important perishable staple diets. Among the tropical root crops, yams provide food for about 400 million people [1]. Moreover, yams are good sources of medicinally important metabolites like diosgenin [2], dioscorin [3] and antioxydants [4]. Some yams are also used as medicines in oriental countries to prevent diabetes [5]. Thus, yams are considered to be useful to human health and they also have nutritional superiority when compared with other tropical root crops. The food species of yams do not produce viable seeds, with some exceptions [6]. Yam is thus propagated vegetatively, generally using fragments of tubers or bulbils [7]. The production of yams is seasonal, so storage is necessary before planting and for use as food. Harvesting is done 180 days after planting [8]. After harvest, tubers enter into dormancy, which is of major importance in yam storage. Once sprouting occurs, storage is no longer possible. Even though the yam is cheaper and of superior quality than other products, the production is hindered by storage problems [9,10].
The high water content of the tubers, combined with damage occurring during or after harvesting, makes them vulnerable to micro-organisms. In the yam tuber, sprout initials are formed beneath the periderm just prior to breakage of dormancy [11]. The sprouting process accelerates the losses and limits storage life.
Dormancy in underground tuber of the Dioscoreaceae is an important adaptive mechanism that ensures that tubers germinate at the start of the growing season. It is also useful to maintain organoleptic quality during storage. Plant breeders are especially keen to manipulate the duration of the dormant period in order to synchronize growth periods and, therefore, to produce more than one generation per year. The control of tuber dormancy, however, is poorly studied and understood.
Tuber dormancy is an important mechanism for adaptation of yams (Dioscorea spp) to their natural environments [12]. Because yams are purchased predominantly as fresh tubers for preparation, long dormancy is very important for shelf life. Hence, long dormancy of tubers is a desirable attribute in yam breeding programs [13]. It permits a better storage but it complicates the use of the tuber for propagation. Irrespective of when a yam seed tuber is planted, the critical starting point of the growing season is when dormancy ends, sprouts are produced. There is a period of about four months after harvest during which tuber losses are incurred in storage but propagation of the planting material would not be successful. In some environments, early planting could largely obviate losses incurred during seed-yam storage if there was more flexibility in the control of the sprouting date through effective means of artificially terminating dormancy. Many workers used plant growth regulators to break or to prolong dormancy but results are inconsistent [10,14].
The aim of the present study was to determine the best conditions for storage of yam microtubers and further sprouting. These conditions can be helpful for their use as “seeds” directly in soil by the growers. However, because of their fragility, they will be more probably transferred to the greenhouse or nursery. This alternative can be necessary to preserve this material with high added value.
To optimize the use of microtubers for the culture in greenhouse or in the field by the farmers, it is important to master the parameters such as the duration of storage and the delay of further sprouting. The present work describes the incidence of the storage duration, the conditions of humidity, temperature, and luminosity during storage and of the size of microtubers on sprouting delay and sprouting rate. This study should help the development of a method for a rapid mass production, storage and sprouting of microtubers of the Dioscorea cayenensis–D. rotundata complex.
2 Materials and methods
2.1 Plant material and tuberisation
In vitro cultures of D. cayenensis–D. rotundata complex (Family: Dioscoreaceare) were provided by the Roots and Tubers Transit Centre of CIRAD (France). The identification numbers were clones CTRT 233 and CTRT 234 both coming from two different tubers of cv. ‘Singo’. Axillary shoot proliferation was maintained by subculturing single nodes, every 2 months on a MS salt medium [15] supplemented with vitamins of Morel [16] and containing 30 g.l−1 sucrose, 2 g.l−1 activated charcoal and 8.2 g.l−1 Caldic agar (Hemiksem, Belgium). The pH of the media was adjusted to 5.7 ± 0.1 before autoclaving at 121°C for 20 min. Cultures were maintained in a 16-h photoperiod (Sylvania Grolux fluorescent lamps, 50 μmol.m−2.s−1) at a day/night temperature of 25/22 °C.
For tuberisation, cuttings (2 cm long) with one leaf were cultured in glass containers (800 ml) with plastic lid containing 125 ml of the same medium [17]. The tubers were harvested for this study after 9 months. Microtubers obtained were then used for storage and sprouting experiments.
2.2 Storage and sprouting of microtubers
For storage, harvested microtubers were kept in 800 ml closed glass jars without medium, five per jar. All treatments consisted of two replicates (at 1 month intervals) with 20 tubers in each. Different storage conditions were tested on the further sprouting of yam Dioscorea cayenenesis–D. rotundata microtubers :
- • day temperature: generally 25 °C but compared with 18 °C in one experiment (Fig. 1);
- • generally in darkness and compared with light (16 h) conditions (Fig. 4);
- • relative humidity: in normal conditions, when tubers were put directly in glass jars, the humidity was around 47%. In some experiments, two layers of filter paper (9 cm diameter) moistened with 3 ml distilled water were laid down in the jars before the tubers. In this case, the relative humidity in the jars was close to 100%;
- • tuber size: generally, only tubers with a length superior to 2.5 cm were used. In some experiments (indicated on figures), microtubers were distributed according to size in three categories: small, medium and large based on the length. Tubers between 1.5–2 cm were considered as small, 2.5–3 cm as medium-sized and above 3.5 cm as large. The tubers below 1.5 cm were rejected;
- • sterility: generally, the glass jars used for storage were previously sterilized and the manipulations were done under sterile airflow. In one case (Fig. 2), the jars were not sterilized and the manipulations were done directly in the lab;
- • duration: from 2 to 18 weeks.
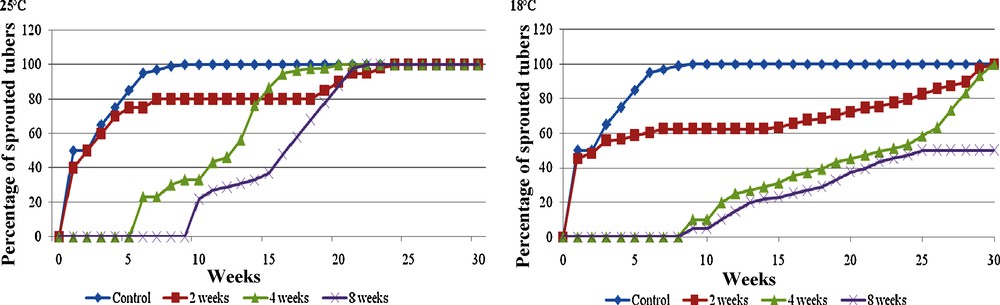
Effect of storage duration in darkness (0 to 8 weeks) at two temperatures (25 or 18 °C) on sprouting of yam microtubers (clone CTRT 233). Control: without storage.
Effet de la durée de conservation (zéro à huit semaines) à l’obscurité et à deux températures (25 °C et 18 °C) sur la germination ultérieure des microtubercules d’igname (clone CTRT 233). Contrôle : sans conservation.
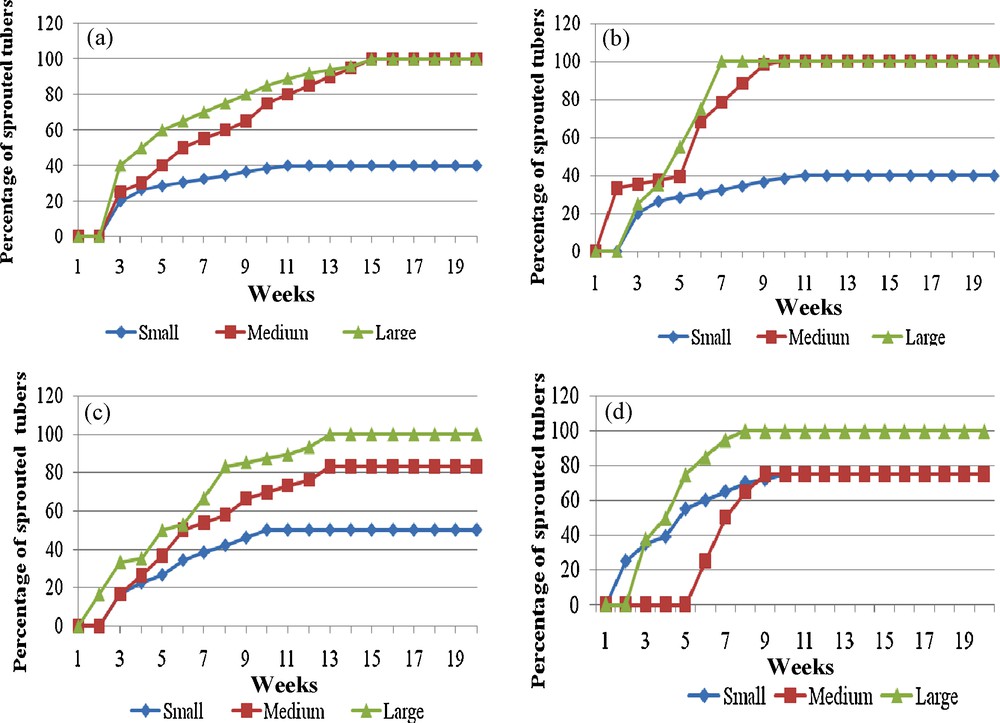
Sprouting after storage (25 °C, 14 weeks) in darkness or light conditions, of different size classes of microtubers obtained on different tuberisation media (Yam clone CTRT 234); a: tuberisation on hormone-free medium, storage in darkness; b: tuberisation on hormone-free medium, storage in light (photoperiod 16 h); c; tuberisation on medium supplemented with 10 μM JA, storage in darkness; d: tuberisation on medium supplemented with 10 μM JA, storage in light (photoperiod 16 h).
Germination après conservation (25 °C, 14 semaines) à l’obscurité ou à la lumière de microtubercules de différentes tailles obtenus sur des milieux de culture différents (clone CTRT 234) ; a : tubérisation obtenue sur milieu sans hormone, conservation à l’obscurité ; b : tubérisation obtenue sur milieu sans hormone, conservation à la lumière (photopériode de 16 heures) ; c : tubérisation obtenue sur milieu additionné de JA 10 μM, conservation à l’obscurité ; d : tubérisation obtenue sur milieu additionné de JA 10 μM, conservation à la lumière (photopériode de 16 heures).
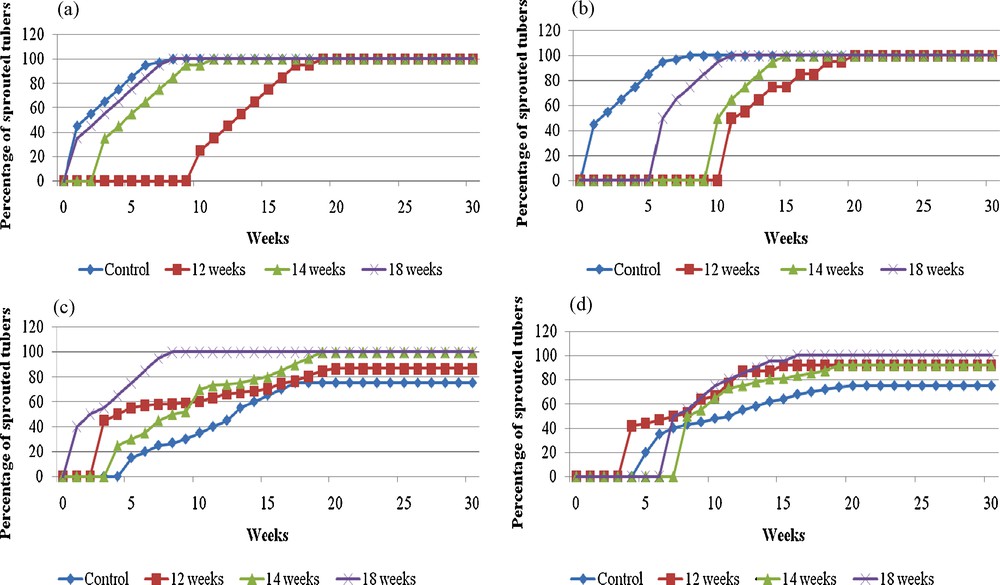
Effect of storage duration (0 to 18 weeks), in sterile and non sterile conditions on sprouting of yam microtubers (clone CTRT 233) at 25 °C in darkness: a: in sterile jars, in vitro sprouting; b: in non-sterile jars, in vitro sprouting; c: in sterile jars, sprouting in sterile compost; d: in sterile jars, sprouting in non-sterile compost.
Effet de la durée de la conservation (zéro à 18 semaines), en conditions stériles et non stériles sur la germination ultérieure des microtubercules d’igname (clone CTRT 233) à 25 °C à l’obscurité : a : dans des récipients stériles, germination in vitro ; b : dans des récipients non stériles, germination in vitro ; c : dans des récipients stériles, germination sur compost stérile ; d : dans des récipients stériles, germination sur compost non stérile.
Tubers were transferred to MS medium without hormones for sprouting in the conditions used for proliferation directly (control) or after several weeks of storage. In some cases, sprouting was compared in sterile and non-sterile composts in a greenhouse under a relative humidity close to 100% during the two first weeks. The temperature of the greenhouse was 26 °C during the day and 22 °C at night.
Sprouts occurred at the head portion of Dioscorea tubers around the point of detachment from the mother plant. Sprouting rates were observed every 7 days. When one bud reached a length of 2 mm, tuber was considered as sprouted
Data represents means of 20 microtubers per treatment, repeated two times.
3 Results and discussion
To use in vitro yam microtubers as seeds, it is important to be sure that all the tubers are able of sprouting. In vitro production can be done the year round but the seeding has to take into account seasonal changes. Meanwhile, microtubers must be stored. However, as for conventional tuber cultures, microtubers could present a dormancy period. The mechanism of dormancy in yam tuber, and for that matter in other tuberous species too, is poorly understood and so the physiological dormancy period cannot be easily predicted or manipulated [18]. Long dormancy of tubers is a desirable attribute in yam breeding and selection programs [13]. It permits a better storage but it complicates the use of the tuber for propagation. Many factors can influence and modify this dormancy.
3.1 Are harvested yam microtubers dormant?
When microtubers were harvested and directly transferred on a new medium without hormones, the tubers rapidly sprouted. After one week, around 50% of the tubers had sprouted and after 8 weeks, all the tubers had sprouted (Fig. 1, 25 °C). Dormancy in traditional culture is widely assumed to start at or shortly after tuber maturity and most studies begin measuring dormancy time from harvest. However, various studies showed that in fact field tubers are dormant well before harvest [12,19]. In the case of the present microtubers harvested after 9 months of in vitro culture, no dormancy was observed. In these conditions, is it possible to store the microtubers?
3.2 Various factors were studied concerning their storage and their further sprouting
3.2.1 Storage duration
The first question was the influence of the storage duration on the further sprouting. The microtubers used in our experiments did not present dormancy if they were transferred in vitro on a new medium directly after harvest. On the contrary, if the microtubers were stored at 25 °C, sprouting was delayed. The longer the storage time, up to 8 weeks, the longer was the delay (Fig. 1). In the case of 8 weeks storage, a delay of 9 weeks was needed before observing the first sprouting and 22 weeks were needed to observe 100% of sprouting. The delay of sprouting was quite similar after 12 weeks of storage (Fig. 2a). In this case, no sprouting was observed before 10 weeks and 18 weeks were needed to obtain the sprouting of all the tubers. When the storage was longer, the delay of sprouting decreased and after 18 weeks of storage, the sprouting was very rapid. Around 35% sprouting was observed after 2 weeks and after 8 weeks, all the microtubers have sprouted (Fig. 2a). It seems thus that storage induced a “dormancy-like period” and this dormancy was maximum between 8 and 12 weeks of storage, i.e. the start of sprouting and the time needed to observe 100% sprouting were maximum. After 18 weeks of storage, the results were reversed, almost similar to the control without storage, only one week of delay. A “dormancy-like” state was induced progressively between 2 and 8 weeks of storage (Fig. 1). Its duration (storage time + sprouting delay) was between 20 weeks for the first tubers to sprout and 28 weeks for the slowest ones. A so “fixed” dormant period (culture time + delay of sprouting) was already observed by Okali [20] in traditional yam culture. The “dormancy like” phase observed can be very useful for the growers of yam tubers to fine-tune more precisely their production depending on the meteorological, agronomical or economical events. Further, it will be interesting to check if a longer storage is possible.
3.2.2 The storage temperature
This has a significant effect on dormancy and the delay of sprouting in traditional culture [21]. Twenty-five degree Celsius was compared to 18 °C, two temperature easily used in Africa. The use of a lower temperature in such countries would still increase the price of the microtubers. The reduction of the storage temperature for microtubers from 25 to 18 °C induced an increase of the delay needed to observe a high percentage of tuber sprouting (Fig. 1). More than 24 weeks were needed when the tubers were stored 2 or 4 weeks at 18 °C while all the tubers stored at 25 °C showed sprouting after 20 weeks. After 8 weeks of storage at 18 °C, the difference was still more important, only 50% of tubers sprouted after 28 weeks. Thus, the delay for sprouting of the microtubers would be longer with the decrease of the storage temperature. These results, showing an increase of the “dormancy like period” with a decrease of temperature, were in agreement with those obtained by [22] in traditional culture. Tubers stored at 16 °C remained dormant for between 120 and 150 days longer than those stored at 21 to 32 °C. For in vitro potato tubers, similar effects of lowering results with storage temperature were observed and a role for endogenous growth regulators as cis-zeatin and cis-zeatin riboside [23], starch and amylose contents [24,25] was shown in the regulation of dormancy. Decreasing storage temperature could be a means to increase yam microtuber dormancy and so to increase storage time. However, these tubers showed a more heterogeneous sprouting, which is not a desirable characteristic for field cultivation. The use of a lower temperature could be advised in the future to increase the storage duration. In parallel, the emergency of dormancy would have to be studied to obtain a homogenous sprouting. The temperature used in the following experiments was 25 °C.
3.2.3 Sterility
During the storage conditions sterility had also an influence on the sprouting rate (Fig. 2). Compared to sterile conditions, storage in non sterile conditions for 14 or 18 weeks further delayed sprouting. It is possible that the development of pathogens can interfere with the “dormancy-like” breaking process. Sterility of storage conditions could ensure a better reproducibility of results and was used in all following experiments.
3.2.4 The relative humidity
Nije [26] has shown the importance in controlling the water content of yam during storage of tubers used from traditional culture. Two different relative humidities in the closed jars used for tuber storage were tested on their further sprouting. In the previous experiments, the humidity in the jars varied between 45 and 50% during the storage. We tested a relative humidity higher than 95% during storage. No significant difference was found on the sprouting rate after storage in the two conditions (Fig. 3). This result is in agreement with the observations of Akoroda [27] on tubers from traditional culture. The process of drying and hardening the surface skin of tubers prior to storage did not have any effect on to the time elapsed until sprouting. On the contrary, in D. spicufolia, storing tuber segments (from traditional culture) at 32 °C and with a low relative humidity delayed sprouting relative to tubers stored at the same temperature with high relative humidity [28]. This difference can be related to the cutting of the tubers of D. spicufolia and, hence, a difference in the loss of moisture for the tuber.
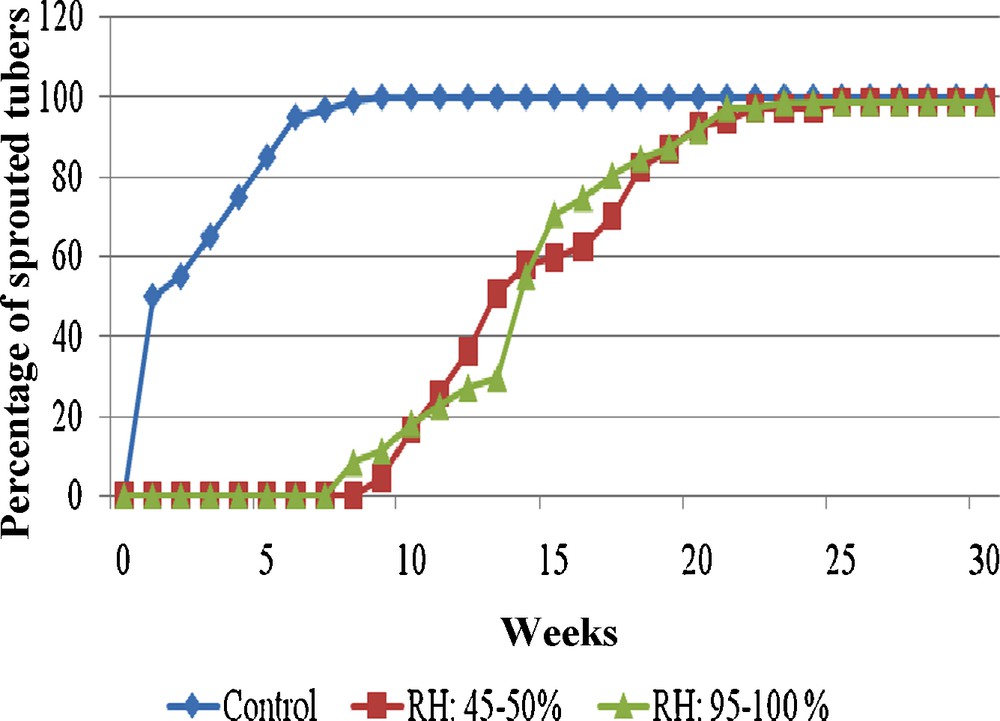
Effect of storage (25 °C, darkness, eight weeks) in two relative humidity conditions (45–50% and 95–100%) on sprouting of yam microtubers (clone CTRT 233). Control: without storage.
Effet de la conservation (25 °C, obscurité, eight semaines) sous deux conditions d’humidité relative (45–50 % et 95–100 %) sur la germination ultérieure des microtubercules d’igname (clone CTRT 233). Contrôle : sans conservation.
3.2.5 The microtuber size
Already in 1988, Alsadon et al. [29] have shown that larger microtubers of potato were able to produce a larger crop than small ones when they were transplanted or directly sown into the field. In yam, the effect of microtuber size before storage was also very important for the further sprouting (Fig. 4). The sprouting of large microtubers (length above 3.5 cm) occurred earlier than that of the smaller tubers (length between 1.5 to 2 cm) sprouted (Fig. 4a). In addition, the final sprouting rate was only 40% for the smaller tubers. The quality of seed potato depends on the starch content, which is related to sprouting vigor [30]. Bigger microtubers had more reserves and thus could more easily sprout, although the dormant period was generally associated with a minimum of endogenous metabolic activity, resulting in very little loss of storage reserve. These results also confirmed those of Vakis [31], who showed that as field tubers became physiologically older (= large) the seeding was faster. When microtubers were physiologically young (= small), sprouting was slower. Then, by aging, their germinal vigor increased and, consequently, the seeding was accelerated.
3.2.6 Light
When stored in the light (Fig. 4b), the sprouting rate of the biggest and medium-sized microtubers occurred earlier that for dark stored tubers. 100% sprouting was observed after 15 weeks if the microtubers were stored in darkness (Fig. 4a) but after 10 weeks if stored in the light (Fig. 4b) (except for the smallest tubers showing only 40% sprouting in both conditions). Our results are in agreement with indications given by Vecchio et al. [32] and Estrada et al. [33] who noted a lengthened dormancy period when potato microtubers were produced in the dark and a reduced time of sprouting with long photoperiodic conditions during the storage phase. Mozie [34] reported similar results on yam tubers (from traditional culture). Nevertheless, storage in darkness was a better solution because it resulted in a more homogenous 100% sprouting, for the medium-sized and the large tubers. These storage conditions are more practical for producers. The smallest tubers should not be used.
3.2.7 Microtuberization conditions
Previously, we have shown that sucrose level incorporated into the tuberisation medium affected the sprouting of the yam microtuber [35]. Tubers obtained on reduced sucrose level sprouted later (without storage). On the other hand, jasmonic acid was often used to promote tuberisation in yams [36–38]. In D. cayenensis–D. rotundata, exogenous application of jasmonic acid positively affected the precocity and the growth of the microtubers [39]. The sprouting of microtubers obtained in the presence of 10 μM jasmonic acid was compared to these obtained on a hormone free medium (Fig. 4). In this case, the sprouting was also earlier when the tubers were stored in the light (Fig. 4d) than in the dark (Fig. 4c) but we obtained 100% sprouting only for the largest tubers. The medium-sized tubers sprouted at around 80% in the light or in the dark, while the sprouting of the smallest ones was improved when the microtubers have been obtained without jasmonic acid. The production of tubers on a medium supplemented with jasmonic acid had not the same effect as exogenous treatment of the tuber. Such a treatment during the storage can prolongate dormancy of D. alata tubers [38]. In our case, we observed the reverse; the delay of sprouting seemed reduced when the microtubers were obtained on medium supplemented with JA.
3.2.8 Ex vitro sprouting
To have practical implications, it was necessary to transfer this technique to standard soil. After storage, the microtubers were transferred in the greenhouse for sprouting (Fig. 2c, d). Freshly harvested microtubers began to sprout after 4 weeks when they were transferred in sterile or non sterile compost while in vitro the first sprouting was observed after one week (Fig. 2a). Moreover, in compost, only 75% of these microtubers sprouted. After 12 weeks of storage, the sprouting was earlier in sterile or non sterile compost than in in vitro conditions. The “dormancy-like” state observed after 12 weeks storage in in vitro conditions was broken more rapidly in compost. After 18 weeks storage, the sprouting in sterile compost was similar to that observed in in vitro conditions: around 40% sprouting after one week and 100% after 8 weeks. In non sterile compost, the delay was more important but 100% sprouting was also observed. Thus, ex vitro sprouting was possible without special difficulties.
4 Conclusion
In these studies, interesting results were obtained when microtubers were harvested after 9 months of culture and were kept dry in jars in sterile conditions during 2 to 18 weeks before sprouting in in vitro conditions. A “dormancy like” phase was observed after 4 weeks of storage, its duration was between 20 and 28 weeks. The size of the tubers used for the storage had great importance for further sprouting. Light during storage had no important effect on the sprouting while a storage temperature of 25 °C permitted a quicker sprouting than 18 °C. The medium used to obtain microtubers can have an effect on sprouting rate. The addition of jasmonic acid in this medium does not have the same effect as its use during storage.
Ex vitro sprouting was not a problem. The delay for sprouting was more important in contrast to in vitro conditions but a rate of 100% was reached. This fact was very important for an agronomical application of this technique for the production of “seeds” (microtubers) directly usable in the field. If the microtubers are too fragile, they can go through a first round of culture in greenhouse or in nursery as currently done for other tubers such as potatoes.
Acknowledgments
Dr Filloux from Cirad for providing the material, Gabon government for awarding scholarship to PO and the skilful assistance of the APE personnel (provided to Cedevit by the government of Wallonia).


