1 Introduction
Community ecologists have long sought to apprehend the various processes shaping coexistence and diversity and to distinguish the relative role of stochasticity and deterministic factors driving species’ responses to environmental and spatio-temporal gradients [1–3]. A large amount of intensive effort focused on quantifying differences in resource use between species [4–6] as identifying resource exploitation patterns can indicate potential competitive interactions and provide information relevant to habitat management. When food is abundant, different bird species may coexist if no other limiting factors are present [7] but if resources are limited, interspecific competition between sympatric species and differences in their ability to exploit these resources can lead to resource partitioning through changes in diet, feeding behavior and spatio-temporal segregation [8,9]. Predator–prey relationships have also been the focus of much attention to interpret resource partitioning and niche distribution in ecosystems [10].
The study of the foraging ecology of sympatric wading birds and the extent of their segregation along niche axes has attracted considerable interest but this sustained effort has not achieved a consensus [11–17]. Water level is known to be a major factor regulating the abundances of aquatic birds and it may determine spatial segregation of bird species together with other factors like plant distribution and cover [18,19]. Information on the species’ ecological requirements and community processes such as competition [20,21] and predation [22–24] is also useful for planning and ensuring the conservation and management of aquatic ecosystems [25].
In order to identify patterns of food use by wading birds and their relationships with respect to their distribution in wetlands, a study on diet components of six aquatic bird species was carried out in Numidia wetlands (NE Algeria) during the breeding season of 2003–2007, analyzing food characteristics and other behavior aspects. The objectives of this study are twofold: (1) document how odonata are used by breeding herons and ibis; (2) identify patterns of relationships between food and habitat use by aquatic birds.
2 Methods
Numidia, northeast Algeria, houses a large and diverse set of wetlands made up of freshwater shallow lakes, ponds, lagoons and brackish marshes [26,27]. The region encompasses a Biosphere Reserve, the El Kala National Park, Ramsar sites and numerous Important Bird Areas [28]. Numidia is also recognized as a national and regional hotspot for Odonata [29]. Between 2003 and 2007, we studied reproductive and diet characteristics of six species of herons, egrets and ibis: Cattle Egret (CE), Ardea ibis L.; Squacco Heron (SH), Ardeola ralloides Scop., Little Egret (LE) Egretta garzetta L., Black-crowned Night Heron (NH), Nycticorax nycticorax L., Purple Heron (PH), Ardea purpurea L. and Glossy Ibis (GI), Plegadis falcinellus L. during the breeding season [30–32].
Food boluses regurgitated by nestlings were collected across the wetland complex of Numidia, northeastern Algeria: Lake Tonga, Lake Fetzara, Dakhla, Chatt, Mekhada, Sidi Achour [28] between May and July during the years 2003–2006. Prey samples were kept in 70% ethanol and were later sorted and identified in the laboratory. Only the adults and larvae of the following broad taxonomic categories of the order Odonata: Zygoptera, Libellulidae and Aeshnidae, were considered in this study. Chicks of all species of herons and Glossy Ibis were individually marked, measured and weighed between 1–23 days [33]. To measure the degree of overlap of two utilization curves (px,i) and (py,i) (relative use of resource categories by each species), we used the Levins index [34]:
During March–April 2006 at Lake Tonga and Lake Oubeïra, one focal bird was chosen at random [36] and its foraging behavior (microhabitat, water level, number of steps, trials, successful catches, intra- and interspecific antagonistic behavior) was recorded for 15 minutes. Only interspecific behavior will be dealt with in this paper.
A data matrix (six species of birds/six categories of prey) was assembled and analysed using Principal Component Analysis (PCA) with the ade4TkGUI package implemented in R [37,38].
3 Results
3.1 Breeding phenology
Despite a great variation and overlap in the timing of the reproduction of the six studied waders, a clear pattern still emerges with the Purple Heron nesting first followed by the Black-crowned Night Heron. The Little Egret and the Cattle Egret then follow with the Squacco Heron nesting last [33]. There is however a great amount of variability in the egg laying period exhibited by the studied herons and ibis. The length of the breeding period is linked to the number of asynchronous nucleii within a colony (Table 1). Egg laying for the Purple Heron is generally over by the end of May whereas the Glossy Ibis has a somewhat protracted egg laying period stretching from mid-April to July [31,32].
Egg laying phenology of Purple Heron, Glossy Ibis and Squacco Heron in Numidia.
| Species | Site and year | First egg date | Egg laying period (days) |
| Purple Heron | Tonga 2004 | < 14 April (several nucleii) | 36 |
| Tonga 2005 | 31 March | 45 | |
| Dakhla 2006 | 31 March | 35 | |
| Fetzara 2006 | 5 April | N/A | |
| Glossy Ibis | Tonga 2004 | ∼ 15 April (several nucleii) | ∼ 75 |
| Tonga 2005 | ∼ 10 May | ∼ 15 | |
| Chatt 2007 | 23 April | N/A | |
| Fetzara 2007 | 30 May | N/A | |
| Squacco Heron | Tonga 2004 | 9 May (several nucleii) | 83 |
| Tonga 2005 | 2 May (two nucleii) | 24 | |
| Chatt 2006 | 5 May | 20 | |
| Fetzara 2006 | 19 May | 23 | |
| Dakhla 2007 | 7 May | 38 | |
| Fetzara 2007 | 16 May | 25 | |
| Cattle Egret | Tonga 2004 | 21 April (several nucleii) | 95 |
| Tonga 2005 | 25 April (two nucleii) | 42 | |
| Chatt 2006 | 25 April | 22 | |
| Fetzara 2006 | 14 May | 28 | |
| Fetzara 2007 | 15 May | 25 | |
| Little Egret | Tonga 2004 | 24 April (several nucleii) | 86 |
| Tonga 2005 | 28 April (two nucleii) | 35 | |
| Chatt 2006 | 28 April | 20 | |
| Fetzara 2006 | 15 May | 22 | |
| Fetzara 2007 | 16 May | 23 | |
| Black-crowned Night Heron | Tonga 2004 | 8 May (several nucleii) | 68 |
| Tonga 2005 | 20 April (two nucleii) | 43 | |
| Mekhada 2005 | 15 May | 29 | |
| Fetzara 2007 | 8 May | 29 |
3.2 Diet and interspecific antagonistic behavior
Odonata were incorporated frequently (> 25%) in the diet by five of the studied species and, for four of these, Odonata represented over 10% of prey items in their diet (Table 2). Size (energy content) seems to be relevant to prey consumption as Odonata larva were fed upon increasingly by more species as size increased (Fig. 1). In sharp contrast, Zygoptera (larva and adults) were mainly preyed upon by small-sized predators. High values of overlap of the utilization curves as measured by the Levins index were found between the larger species (Purple Heron, Black-crowned Night Heron and Glossy Ibis) and the smaller species (Squacco Heron, Little Egret and Cattle Egret), respectively (Table 3). Out of 20 interspecific agonistic encounters recorded, 11 involved the studied species, particularly the small-sized species: Squacco Heron, Little Egret and Cattle Egret (Table 4).
Occurrence and number of Odonata prey items in the diet of herons and ibis nestlings’ diet.
| Occurrence (%) | Number (%) | Food boluses | Prey items | |
| Cattle Egret | 27.8 | 5.0 | 243 | 4991 |
| Little Egret | 30.0 | 10.4 | 603 | 603 |
| Squacco Heron | 54.3 | 31.0 | 71 | 591 |
| Black-crowned Night Heron | 8.3 | 3.0 | 24 | 76 |
| Glossy Ibis | 42.1 | 13.0 | 21 | 169 |
| Purple Heron | 32.0 | 15.8 | 100 | 399 |
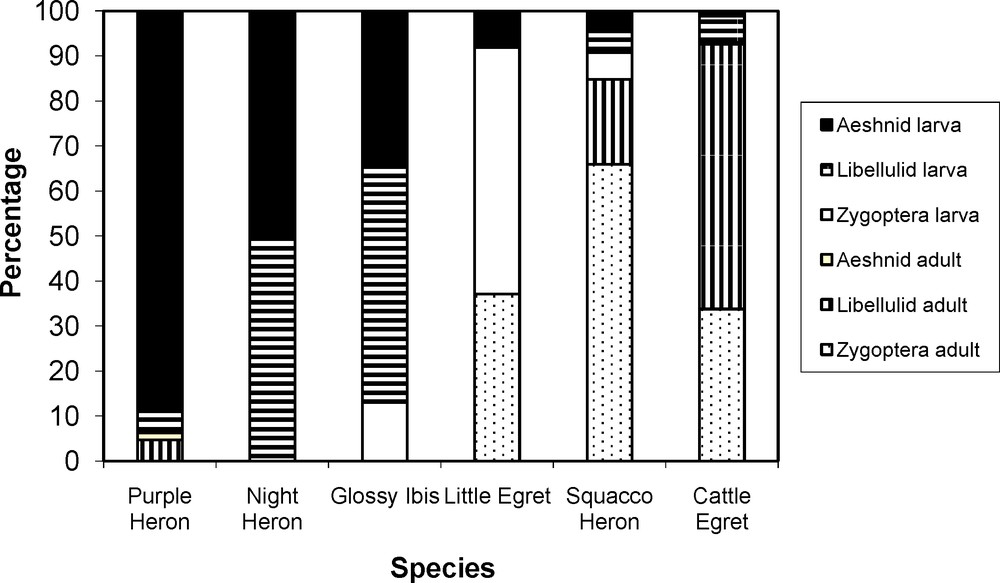
Relative importance of Odonata in the diet of herons and ibis in their choice of Odonata.
Levins index for overlap of herons and ibis in Numidia based on odonata as prey. Data correspond to overlap of species on each row with species on the columns.
| Species | Purple Heron | Night Heron | Little Egret | Squacco Heron | Cattle Egret | Glossy Ibis |
| Purple Heron | 1 | 0.59 | 0.09 | 0.06 | 0.18 | 0.42 |
| Night Heron | 0.94 | 1 | 0.08 | 0.09 | 0.07 | 0.87 |
| Little Egret | 0.16 | 0.09 | 1 | 0.63 | 0.28 | 0.22 |
| Squacco Heron | 0.09 | 0.10 | 0.59 | 1 | 0.71 | 0.1 |
| Cattle Egret | 0.32 | 0.08 | 0.27 | 0.73 | 1 | 0.08 |
| Glossy Ibis | 0.82 | 1.06 | 0.24 | 0.12 | 0.08 | 1 |
Number of recorded interspecific agonistic encounters between different species of herons and ibis.
| Species | GE | LE | GH | WS | CE | PH | SH | NH | GI | Total |
| Great Egret (GE) | 0 | |||||||||
| Little Egret (LE) | 0 | |||||||||
| Grey Heron (GH) | 5 | 2 | 7 | |||||||
| White Stork (WS) | 1 | 1 | ||||||||
| Cattle Egret (CE) | 1 | 1 | ||||||||
| Purple Heron (PH) | 0 | |||||||||
| Squacco Heron (SH) | 5 | 1 | 3 | 9 | ||||||
| Black-crowned Night Heron (NH) | 0 | |||||||||
| Glossy Ibis (GI) | 2 | 2 | ||||||||
| Total | 5 | 8 | 2 | 0 | 5 | 0 | 0 | 0 | 0 | 20 |
3.3 Multivariate analysis of diet
Despite some overlap in the use of prey species, results indicated a clear pattern of feeding segregation amongst nesting herons by their differential exploitation of different taxa or stages of Odonata which differ markedly in their size (biomass) and habitat use (Fig. 2). The factorial plane of the first two axes of the PCA (79% of inertia) revealed the structure of the data marked by a size dichotomy along the first axis between large species (Purple Heron, Black-crowned Night Heron and Glossy Ibis) which prey on large preys like the Aeshnids and small predators which feed mainly on Zygoptera adults and larvae. The second axis reveals a gradient of habitat use from terrestrial to aquatic ones illustrated by Cattle Egrets that feed on adult Libellulids which can disperse far from water, Squacco Herons which mainly collect adult Zygoptera on the margins of ponds and lakes, and Little Egrets which wade in shallow water to prey on Zygoptera, both adults and larvae.
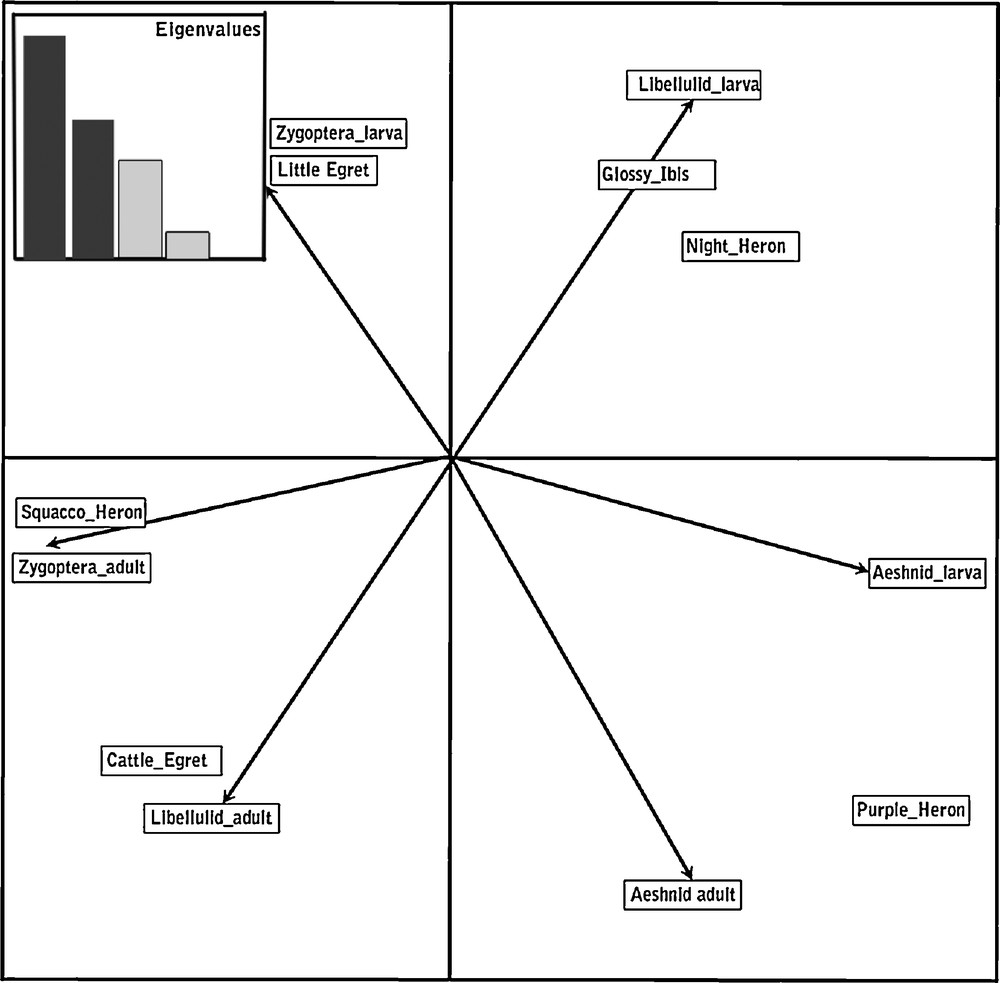
Biplot of factorial plane 1 × 2 of PCA of the food of herons and ibis’ nestlings. Eigenvalues are provided in the insert.
The third axis (16% of inertia) indicated that within the set of large predators, Purple Herons specialize on the largest preys, Aeshnid larvae, close to emergent vegetation, whereas Glossy Ibis feeds on Libellulid larvae in more open habitats (Fig. 3). The limited data on the nocturnal Black-crowned Night Heron suggest a diet intermediate between the latter two species. The fourth axis (5% of inertia), separated the Squacco Heron which feeds more on adult Zygoptera from the Little Egret which specializes on larval Zygoptera.
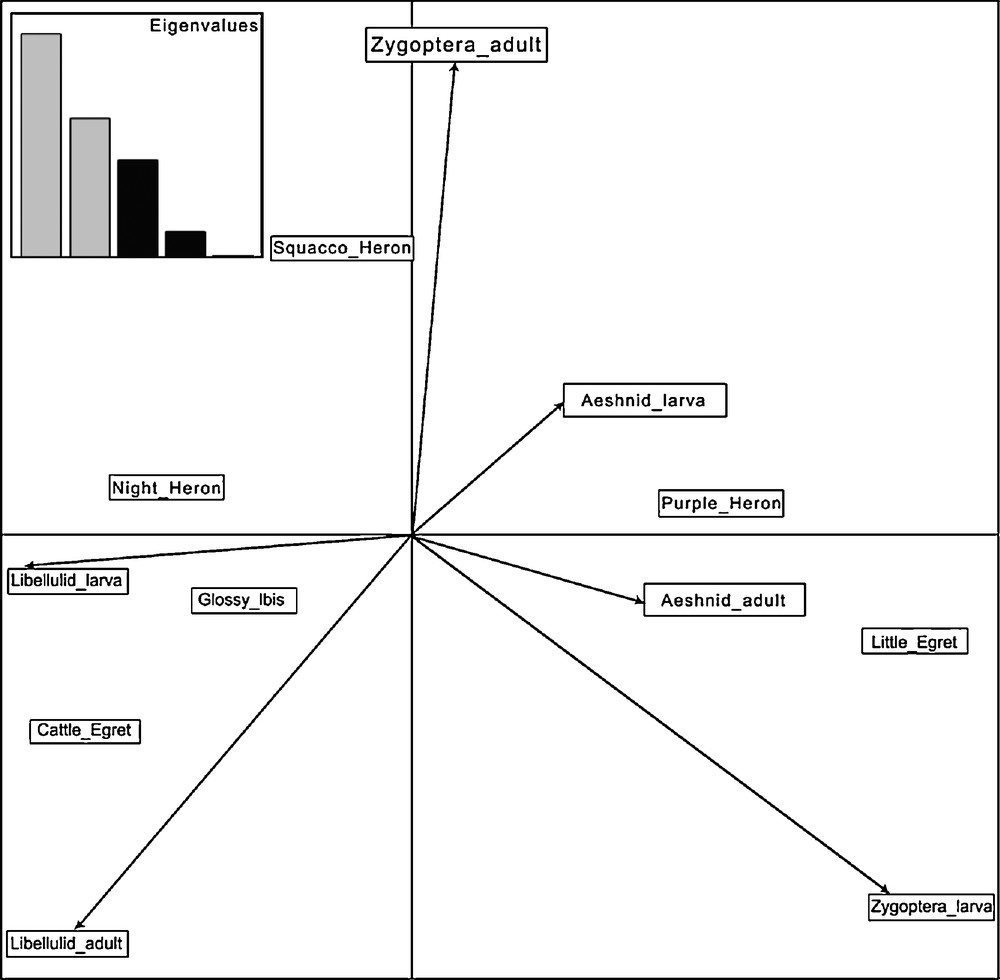
Biplot of factorial plane 3 × 4 of PCA of the food of herons and ibis’ nestlings. Eigenvalues are provided in the insert.
4 Discussion
Reproductive success is tightly linked to foraging success [39]. In two seminal papers [40,41], Hutchinson described the degree of limiting similarity among coexisting, congeneric species based on morphological character divergence. Hutchinson's rule predicts that species that differ sufficiently in size or life history traits, may also differ in resource use to avoid competitive exclusion. The rule also implies that niches of herons and ibis in Numidia are spaced in a non-random way in Hutchinson's hypervolume with minimal overlap in at least some dimensions [4,42,43]. The link between niche overlap and competition can be fairly complex [44] and much theoretical and experimental effort has been devoted to finding mechanisms explaining coexistence of competing species [5,45]. Resource competition is acknowledged as one of the most important processes that structure ecological communities [46] and, likewise, predation is also widely accepted as a major structuring force that may affect prey populations by influencing behavior and habitat use [47,48]. A greater knowledge of resource competition and predation as well as mechanisms underlying these important processes and their interplay is required for the conservation of vanishing colonial waterbirds in a water-stressed region facing global changes [49].
The reproductive period is a critical period in the birds annual life cycle, when trophic resources may be limited and geared to meet the specific needs of developing chicks [50]. Resource competition at this stage may affect vital rates (e.g. birth and survival) and influence population dynamics. However, interspecific competition is relaxed through differences in the timing of reproduction of sympatric species which staggers to some extent the peak of energy demands of developing chicks [51]. Temporal partitioning may thus facilitate coexistence between competitors in ecological communities [52,53]. The studied species (both prey and predators) show a sequential distribution of their breeding time in accordance with similar observations of the same species in other geographic zones [54–56]. Chick development is closely linked to chick provisioning which in turn is linked to food availability and rate of parental provisioning [57,58]. Diel temporal partitioning may also mediate coexistence between predators and prey but the detailed diurnal time budget of local herons and ibis deserves further investigation. In the study area, the Black-crowned Night Heron is the only nocturnal wader although diurnal foraging is carried out by breeding pairs at the height of the reproductive season [59]. Perhaps, significantly, the emergence of Aeshnids, a time of high vulnerability, is carried out before dawn, at a time when most avian predators are inactive. Both Aeshnids and Libellulids, which are also strong flyers, are highly vulnerable when flying in tandem during copulation and egg laying.
Another way to lessen competition between the studied herons and ibis is through the use of a wide range of feeding methods, probably aimed at distinct types of prey [39]. Purple Herons uses the ‘stand and wait’ [51,60] in relatively deep waters to capture prey (odonata, fish and other taxa) too large for any other heron and ibis to handle and, as expected from the analysis of his diet, this large heron hardly interacts with smaller-sized waders. The Glossy ibis forages in groups by probing the substrate in shallow waters and although, like the Black-crowned Night Heron, it preferentially incorporates Libellulid larvae in its diet, it can hardly be considered a strong competitor to a mainly piscivorous guild. Antagonistic interactions have been noted between the Glossy Ibis and Cattle Egret when the two overlap in the same microhabitat (wet open pastures). Direct interference competition seems to be concentrated mainly among the small body-sized set (Little Egret, Cattle Egret and Squacco Heron) with high overlap of their utilization curves. This is especially true for the Little Egret and the Squacco Heron which also share other prey (Mosquito Fish Gambusia holbrooki Girard and amphibians) and which exhibit the highest rate of aggressive interactions. The former uses relatively deep waters and adopts diverse and most active feeding techniques to capture Zygopteran larvae, small fish, crustacea and amphibians. In contrast, the latter favors slow paces along the shallow parts of the shore of wetlands or waits motionless for prey to pass by before striking. This differential use of microhabitats and feeding strategies may help partition resources between the two species as has been found in other wading bird communities [61]. The last species, Cattle Egret, is mainly a terrestrial insect specialist which favors flying odonata which it hunts around dryer margins of waterbodies. Some scattered observations carried out at Lake Tonga and other neighboring wetlands corroborate this as they reveal that the studied species occupy distinct microhabitats with the Cattle Egret foraging mainly on wet and dry pasture lands adjacent to waterbodies; the Squacco Heron is generally found on the margin of wetlands and on floating vegetation within the waterbodies; the most active wader, the Little Egret, forages in shallow, open waters whereas the Purple Heron favors deeper parts in the littoral zone close to reed beds and other emergent vegetation; and the Glossy Ibis is found in wet pastures and open shallow areas whereas the Black-crowned Night Heron, which rarely forages during the day, has been recorded on the banks of canals.
Predators preferentially hunt prey of an optimal size in order to maximize the net rate of energy intake. Hence, prey body size is a trait that has been well documented to play a large role in influencing predator's choice [62,63] and capture of distinct prey length, correlated with the body size of herons, may be one way to achieve resource partitioning [64–66]. However, a positive relation between size of herons and size of prey may not be decisive in niche segregation [67]. In Numidia, size of odonata but also of fish prey (unpub.) varies within the large waders set (Purple Heron, Black-crowned Night Heron and Glossy Ibis) and within that of the small waders set (Little Egret, Cattle Egret and Squacco Heron).
Diet overlap amongst local waders is lessened by resource use of distinct predators on different prey or developmental stages based on body size and habitat use. However, because of the dynamic properties of the niche, more information is needed about resource partitioning by the studied waders when resource levels are reduced and competition heightened [68,69]. The differential exploitation of Odonata may be the result of habitat selection of both prey and predators and further research is needed to explore the spatial distribution of Odonata. Resource partitioning through the differential use of foraging areas and distinct antagonistic behavior deserves further study [61,70,71].
Odonata, at least in numbers, form a sizeable component of the diet of nestling herons and ibis in Numidia, northeast Algeria as elsewhere [72,73] and their utilization suggests a pattern of resource partitioning by nesting herons and ibis similar to that found in southern Florida [12] and Australia [74], but future studies should examine how other taxa (fish, amphibians, etc.) are exploited and attempt to disentangle various interacting processes that shape the composition of ecological communities.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
We are most grateful to two anonymous referees for their instructive comments and KAS Al-Rasheid for his help. This research was supported by the Algerian Ministry of Higher Education and Scientific Research (DGRSTD/MESRS) and the King Saud University Deanship of Scientific Research, Research Group Project No.: RGP-VPP-135.


