The lymnaeid genus Radix Montfort, 1810 has a wide geographic distribution, being represented in waterbodies of Eurasia, Africa, and North America [1–6]. Its type species is Radix auricularia (L., 1758), occurring in the Northern Palaearctic and Nearctic [7]. The highest species richness of Radix is observed in the Middle East, Central and South-Eastern Asia [8], whereas the northern regions of the Palaerctic are inhabited by a few species [6,9]. The practical significance of Radix spp. is explained by the fact that some species of this genus serve as primary hosts for trematode larvae of the species Fasciola gigantica Cobbold, 1855 [8,10]. Despite this practical importance, the species level taxonomy of Radix is still far from being stable. Due to high level of conchological and anatomical variation in Radix, the unequivocal delineation of species in this group is very difficult [11]. Besides, the lymnaeid researchers may follow different species concepts and their opinions on how to delineate species in the Lymnaeidae may thus be drastically dissimilar. The systematics of European Radix spp. gives a striking example of this. Hubendick [7] and Jackiewicz [12,13] accepted only two species of this group in Europe, Lymnaea (Radix) auricularia and L. (R.) peregra (O.F. Müller, 1774), characterized by great shell variability and uniform reproductive morphology. The main anatomical distinction between the two species according to Hubendick [7] is the length of the spermathecal duct (long in L. auricularia; short or almost absent in L. peregra). On the other hand, Kruglov and Starobogatov [3,4,14] in Russia believed that these anatomical differences are phylogenetically significant enough to substantiate the splitting of Radix into two subgenera: Radix s. str. (= Lymnaea auricularia sensu Hubendick) and Peregriana Servain, 1881 (= L. peregra sensu Hubendick). On the basis of slight differences in shell geometry, Kruglov and Starobogatov [3,4] distinguished two species of Radix s. str. and 19 (!) separate species of Peregriana in European waterbodies only, not to mention nearly 20 endemic species living in Siberia, Central Asia, and the Russian Far East [8].
Such drastic discrepancies between the two systems apparently result from various interpretations of the same morphological information proposed by different authors that made it crucial to find an additional source of data independent of morphology. The progress of molecular taxonomic techniques provided researchers with such a source, and the current taxonomy of Radix in Europe is based mainly on molecular taxonomic inferences obtained by several independent teams of investigators [11,15–19]. To date, at least five widespread species of European Radix have been characterized molecularly, and their species status is accepted by all modern taxonomists [6,8,9,20]. These five species are as follows: R. ampla (Hartmann, 1821), R. auricularia, R. balthica (L., 1758), R. labiata (Rossmässler, 1835) [= R. peregra auct.], and R. lagotis (Schrank, 1803). Several other species of Radix, being endemics of Southern Europe (Balkans), were also studied in this respect [18].
The taxonomy of the North Asian species of Radix is much more complicated. Several tens of nominal taxa assigned to this genus were described in the 19th–20th centuries from waterbodies of Siberia and of the Russian Far East [14,21–24]; however, their validity was based on morphological traits only and in many cases raised some doubts. The presence of some European species of Radix in Siberia (for example, R. balthica) has been validated by molecular means [11], but we still lack a whole picture of the taxonomic diversity of the genus in this region. It is quite possible to find endemic species here, but the modern taxonomic standards demand the genetic verification of the species status in addition to morphological evidence.
The general aim of this article is to provide present genetic, morphological, and zoogeographical evidence in favour of a hypothesis that there is at least one endemic species of Radix in Siberia (R. dolgini s. lato), in addition to five species of this genus (see above) previously identified molecularly from the Euro-Siberian region [11,19].
1 Material and methods
1.1 Sampling
The samples of Siberian molluscs of the genus Radix were carried out in different waterbodies situated in the Western Siberian plain, Altai region and Northern Baikal area (Table 1, Tables S2 and S3). In this study, we focused on the examination of four species of Radix (Peregriana) described from Siberia.
List of sequenced specimens of Radix dolgini. Materials are deposited in RMBH and SNSD collections.
| Haplotype code | NCBI's GenBank acc. no. | Specimen voucher | Locality | |
| COI | ITS2 | |||
| dol1 | KT030067 | KT030049 | MlymB-61 | Republic of Buryatia: Kironsky geothermal spring, 55°57′17′′N, 110°42′19′′E |
| dol1 | KT030068 | KT030050 | MlymB-62 | |
| dol1 | KT030069 | KT030051 | MlymB-63 | |
| dol2 | KT030070 | KT030052 | MlymB-87 | Republic of Buryatia: Verkhnyaya Angara River, 55°57′34′′N, 110°30′28′′E |
| dol3 | KT030071 | KT030053 | MlymB-88 | |
| dol3 | KT030072 | KT030054 | MlymB-89 | |
| dol3 | KT030073 | KT030055 | MlymB-90 | |
| dol4 | KT030074 | KT030056 | MlymB-91 | |
| dol5 | KT030075 | KT030057 | MlymB-109 | Republic of Buryatia: Verkhnyaya Angara River, 55°57′38′′N, 110°31′24′′E |
| dol5 | KT030076 | KT030058 | MlymB-111 | |
| dol2 | KT030077 | KT030059 | MlymB-112 | Republic of Buryatia: Verkhnyaya Angara River, 55°57′24′′N, 110°30′60′′E |
| dol6 | KT030078 | KT030060 | MlymB-114 | |
| dol7 | KT030079 | KT030061 | SNSD Moll S5217 | Altay Territory: a swamp in the floodplain of Kulunda River, 50°59′50′′N, 80°00′07′′Eb |
| dol8 | N/Aa | KT030062 | SNSD Moll S5218 | |
| dol9 | N/A | KT030063 | SNSD Moll S51130 | Novosibirsk Region: a swamp alongside Severnoye–Biaza road, 56°25′25′′N, 78°17′15′′Ec |
| dol10 | N/A | KT030064 | SNSD Moll S52861 | Tomsk Region: a lake near Novoshumilovo village, 57°25′30′′N, 88°31′13′′E |
| dol11 | N/A | KT030065 | SNSD Moll S52862 | |
| dol12 | N/A | KT030066 | SNSD Moll S52863 |
a N/A: not available.
b Initially determined as R. gundrizeri.
c Initially determined as R. ulaganica.
1. R. dolgini (Gundrizer and Starobogatov, 1979). Type locality: Russia, Krasnoyarsk territory, a lake in the floodplain of the Kureika River, 20 km upstream of its mouth. The Kureika River is the right tributary of Yenisei River; the coordinates of its mouth are: 66°29′17′′N; 87°14′08′′E). We studied samples of this species from 46 localities containing altogether nearly 650 animals.
2. R. gundrizeri (Kruglov and Starobogatov, 1983). Type locality: Russia, Republic of Altai, a small waterbody (spring) on the shore of Lake Sorulu-Kol’ (approximately 50°27′20′′N; 87°30′10′′E). We possessed two samples of R. gundrizeri (≈ 50 individuals in total) from the Tyumen and Altai regions of Russia.
3. R. kurejkae (Gundrizer and Starobogatov, 1979). Type locality: Russia, Krasnoyarsk territory, a lake in the floodplain of Kureika River, 20 km upstream of its mouth. The type series of R. kurejkae and R. dolgini have the same locality and origin from the same sample collected on 7 July 1978 by V.A. Gundrizer. Fifteen samples from the Tyumen and Tomsk regions that contained about 100 empty shells, determined as belonging to R. kurejkae, were examined.
4. R. ulaganica (Kruglov and Starobogatov, 1983). Type locality: Russia, Republic of Altai, a small waterbody (spring) on the shore of Lake Sorulu-Kol’ (the type series of R. gundrizeri and R. ulaganica have the same locality and origin from the same sample collected 29 July 1978 by E.A. Novikov). We studied 22 samples of this species (≈ 170 individuals in total) from different localities situated in the southern part of Western Siberia.
The snails were sampled by hands or by means of a sieve on shallow zones of waterbodies or from vegetation. The animals were placed directly into 96% ethanol. Their primary identification, measurements and dissections were carried out in the laboratory. Taxonomic identifications of the Siberian endemic lymnaeids were made by using the original descriptions of the species [14,24,25] as well as by consultation with the more recent keys [20,26]. In addition to the samples listed above, we studied the type series of the aforementioned four species that are kept in the collections of the Zoological Institute of the Russian Academy of Sciences, Saint-Petersburg (ZIN). The malacological collection of the Limnological Institute, Siberian Branch of the Russian Academy of Sciences, Irkutsk, Russia (LIN), was also inspected. The voucher specimens of the studied snails are housed in the Museum of Siberian Aquatic Molluscs, Omsk State Pedagogical University, Omsk, Russia (MSAM); Russian Museum of the Biodiversity Hotspots (Institute of Ecological Problems of the North; Ural Branch of the Russian Academy of Sciences, Arkhangelsk, Russia; RMBH); and Senckenberg Natural History Collections, Dresden, Germany (SNSD). The comparative material of other Eurasian Radix species has been examined in the collections of ZIN, of SNSD, of the Natural History Museum of Denmark (Copenhagen; ZMUC), and of the “Museum für Naturkunde”, Berlin, Germany (ZMB).
One hundred twenty-six shells were measured following [8,13]. We measured 14 shells of R. gundrizeri (from locality 32; see electronic Table S2), 27 shells of R. ulaganica (from localities 23 and 29), and 85 shells of R. dolgini (from localities 21, 27, 30 and 36). The six measurements have been taken from each shell: shell height and width, spire height, body whorl height, aperture height and width. All measurements were made with calipers or the ocular-micrometer of a binocular microscope with an accuracy of 0.1 mm. Also, the number of whorls was counted.
The linear measurements alongside with the numbers of whorls were subjected to principal component analysis (PCA) and canonical variate analysis (CVA) in STATISTICA 10.0 for Windows (StatSoft Inc, USA). The initial data were standardized before processing.
Seventy individuals of different species of snails were dissected by us. We examined taxonomically significant parts of the male and female reproductive systems, including the copulative apparatus, the prostate, the bursa copulatrix and its duct. The index of the copulatory apparatus (ICA) was calculated for each dissected individual as the ratio between the lengths of its praeputium and the penis sheath (see [27] for details).
The soft bodies of individuals belonging to all species, except of R. kurejkae, were available for study. Therefore, the molecular analyses were restricted to three other Siberian species of Radix.
The distribution map of R. dolgini s. lato in Siberia is based on localities of this species revealed during examination of ZIN, MSAM, RMBH, and LIN collections. Some literature sources were also taken into account [28,29]. However, we avoided using the distribution data published in non-annotated checklists and other sources that fail to provide accurate taxonomic identifications. The collected geographical data was mapped using ESRI ArcGIS 10; for clarity, closely situated records were merged into single locations (Tables S2 and S3).
1.2 DNA isolation, PCR, and sequencing
The present study includes new molecular data for 18 specimens of R. dolgini (Table 1). Genomic DNA was extracted from individual snails using the Diatom DNA Prep 200 reagents kit (“Laboratoriya Isogen” LLC, Russia) following the manufacturer's protocol. In the present study, we used data inferred from the mitochondrial cytochrome oxidase subunit I (COI) and the nuclear internal transcribed spacer 2 (ITS2) gene fragments, because the simultaneous analysis of the nuclear and mitochondrial markers provides the most representative taxonomic results [30]. A total of 657 base pair (bp) fragment of the COI gene and a 408 or 409 bp fragment of the ITS2 gene were amplified. COI primers were LCO1490 and HCO2198 [31]. For ITS2, the primers LT1 [15] and ITS4 [32] were used. The PCR mix contained approximately 200 ng of total cellular DNA, 10 pmol of each primer, 200 μmol of each dNTP, 2.5 μL of PCR buffer (with 10 × 2 mmol MgCl2), 0.8 units of Taq DNA polymerase (SibEnzyme Ltd., Russia), and H2O, which was added up to a final volume of 25 μL. Thermocycling included one cycle at 95 °C (4 min), followed by 36–39 cycles of 95 °C (50 s), 52 °C (50 s), 72 °C (50 s) and a final extension at 72 °C (5 min). Forward and reverse sequencing was performed on an automatic sequencer (ABI PRISM3730, Applied Biosystems) using the ABI PRISM BigDye Terminator v.3.1 reagent kit. The resulting sequences were checked using a sequence alignment editor BioEdit version 7.2.5 [33].
A total of 68 COI and ITS2 sequences representing 52 lymnaeid specimens were obtained from NCBI's GenBank (Table S1). As ingroup taxa, sequences of 15 species in the genus Radix were sampled, i.e. R. ampla, R. relicta, R. balthica, R. lagotis (deposited in GenBank as R. zazurnensis), R. labiata, R. auricularia, R. rubiginosa, R. quadrasi, R. natalensis, R. sp. “clade 1”, R. sp. “clade 2”, R. sp. “clade 3”, R. sp. “clade 5”, R. sp. “clade 9” and R. sp. “clade 10”. These sequences represented the majority of biological Radix species from Eurasia (i.e. European countries, Russia and some regions of Southeast Asia), the validity of which was confirmed using molecular data, as well as some species with unclear taxonomic names, primarily from China, Nepal and Vietnam. In addition, four sequences of Galba truncatula and Lymnaea stagnalis were obtained as outgroups.
1.3 Sequence alignment and phylogenetic analyses
The alignment of the COI and ITS2 sequences was performed using the ClustalW algorithm implemented in MEGA6 [34]. The aligned sequence datsets were checked with GBlocks v. 0.91b [35] to exclude hypervariable fragments from the sequence alignments using options for less stringent selection, which enabled gap positions, smaller final blocks, and less strict flanking positions. For the phylogenetic analyses, each sequence of the aligned datasets was trimmed, leaving a 600-bp COI and a 371-bp ITS2 fragment. The sequence datasets were collapsed into haplotypes and combined into two-gene dataset using an online FASTA sequence toolbox FaBox 1.41 [36]. The analyses were performed using the resulting combined dataset with unique haplotypes, including two outgroup taxa. The best models of sequence evolution for each partition as suggested based on corrected Akaike Information Criterion for small sample sizes (AICc) of MEGA6 [34] were as follows: (1) 1st codon of the COI: HKY; (2) 2nd codon of the COI: GTR + G + I (I = 0.05, G = 1.68); (3) 3rd codon of the COI: TN93 + G (G = 0.19); (4) ITS2: GTR + G (G = 2.01). The phylogenetic relationships were reconstructed for a combined dataset based on Bayesian inference as implemented in the software package MrBayes version 3.2.3 [37] through the CIPRES Science Gateway [38] using following parameters: nchains = 4, ngen = 5,000,000, samplefreq = 1000, temp = 0.1. Convergence of the MCMC chains to a stationary distribution was checked visually using an MCMC trace analysis tool (Tracer version 1.6 [39]). The resulting trees were constructed using a tree figure drawing tool (Archaeopteryx software v. 0.9901 beta) [40].
The mean distance between R. dolgini and R. labiata COI haplotype groups was calculated using MEGA6 software [34] under the Tamura–Nei model with a gamma distribution of a rate variation among sites. The HKY model, which is the most suitable substitution model for our sequence dataset, is not available in this algorithm, but the Tamura–Nei model actually represents its updated modification [34,41]. In addition, we calculated the distances between all species in the COI gene dataset under Kimura-2-Parameter model to compare with those from other studies.
2 Results
2.1 Molecular phylogeny
The COI distances between Radix species calculated under the Kimura-2-Parameter (K2P) model, which is most commonly used for interspecific comparisons, are presented in Table 2. The mean K2P distance between R. dolgini and other species within the genus varies from 9.8 to 18.3%. According to results of phylogenetic analysis (Fig. 1), the Radix species are distributed between two relatively well-supported basal clades with Bayesian posterior probabilities (BPP) of 0.8–0.89. This dichotomy corresponds well with the proposition to split the genus into two subgenera – Radix s. str. and Peregriana [3,4,42]. The European species of Peregriana constitute two separate clades whose BPP varies from 0.97 to 0.99. The first of these clades includes four species (R. ampla, R. relicta, R. balthica, and R. lagotis), whereas the second one is formed by R. labiata alongside with a host of Siberian endemic species – R. dolgini, R. gundrizeri, and R. ulaganica. The tree shows some signs of geographic pattern, because the specimens collected in the Baikal Region (Republic of Buryatia) are separated from those sampled in Western Siberia (Altai, Novosibirsk and Tomsk regions). The R. labiata clade is a sister group to that containing three Siberian species of Radix. This dichotomous pattern is supported by a high BPP value (0.99) in the tree.
Mean K2P distances (%) between Radix species based on the COI sequencesa.
| Species | R. ampla | R. auricularia | R. balthica | R. labiata | R. relicta | R. sp. “clade 2” | R. sp. “clade 3” | R. sp. “clade 5” | R. sp. “clade 9” | R. sp. “clade 10” | R. lagotis | R. rubiginosa | R. natalensis |
| R. auricularia | 15.9 | ||||||||||||
| R. balthica | 10.0 | 15.6 | |||||||||||
| R. labiata | 12.4 | 17.2 | 13.0 | ||||||||||
| R. relicta | 4.1 | 16.8 | 10.9 | 13.1 | |||||||||
| R. sp. “clade 2” | 17.1 | 10.5 | 17.6 | 16.8 | 17.2 | ||||||||
| R. sp. “clade 3” | 17.8 | 14.8 | 18.3 | 18.0 | 18.6 | 16.1 | |||||||
| R. sp. “clade 5” | 14.1 | 14.9 | 14.5 | 13.2 | 12.7 | 15.8 | 16.1 | ||||||
| R. sp. “clade 9” | 12.1 | 16.3 | 14.2 | 11.9 | 12.5 | 15.0 | 18.8 | 13.3 | |||||
| R. sp. “clade 10” | 14.6 | 17.4 | 14.1 | 11.8 | 14.1 | 15.9 | 19.0 | 14.3 | 8.2 | ||||
| R. lagotis | 10.8 | 15.8 | 9.4 | 11.8 | 11.9 | 17.0 | 18.3 | 13.2 | 12.8 | 13.6 | |||
| R. rubiginosa | 16.2 | 14.1 | 15.9 | 16.9 | 16.2 | 16.1 | 14.1 | 13.4 | 14.8 | 15.0 | 17.3 | ||
| R. natalensis | 14.9 | 12.9 | 15.3 | 15.3 | 14.2 | 15.4 | 14.7 | 12.3 | 13.7 | 15.0 | 16.6 | 4.3 | |
| R. dolgini | 13.4 | 18.3 | 12.0 | 9.8 | 11.8 | 17.1 | 17.2 | 12.3 | 13.0 | 14.0 | 11.2 | 16.0 | 14.4 |
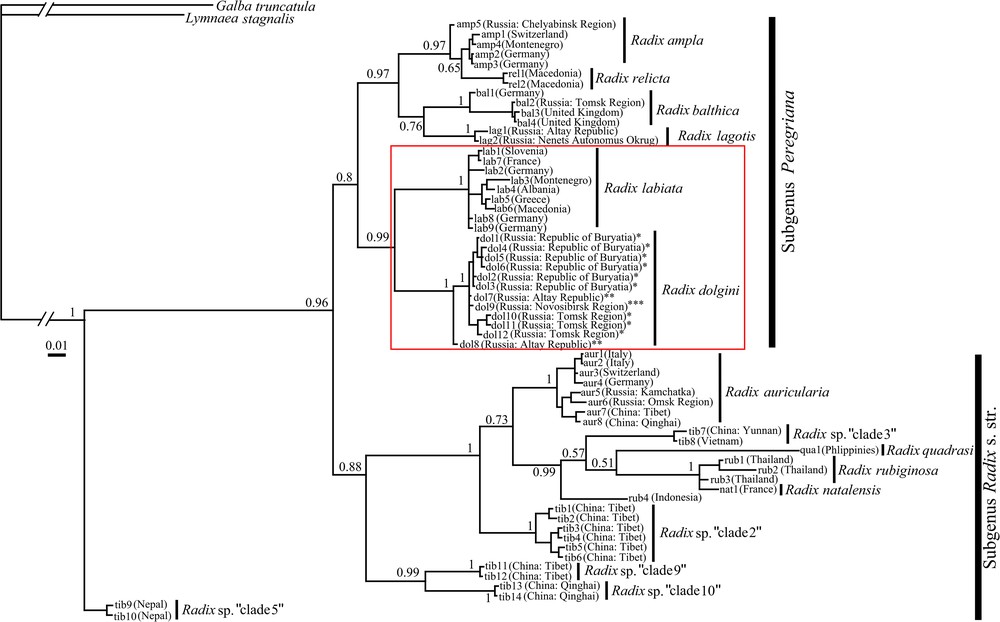
Bayesian phylogram of Radix haplotypes based on combined mitochondrial (COI) and nuclear (ITS2) datasets (Table 1, Table S1). The scale bar indicates the length of the branch. Bayesian posterior probabilities (BPP) are mentioned in the figure. Specimens of R. dolgini were initially identified by morphological and anatomical features as: *R. dolgini; **R. gundrizeri; ***R. ulaganica.
2.2 Morphology
The holotypes of the three Siberian endemic Radix species possess a somewhat similar conchological appearance having a relatively slender shell with a rather high conical spire, a moderately inflated body whorl, and flattened spire whorls (Fig. 2). The shell variation across the three species is prominent (Fig. 3), but all conchological variants may be viewed as parts of the same continuum of intergrading forms that differ from each other by the relative height of the spire or by whorls number, i.e. by quantitative, not qualitative distinctions. We failed to find a hiatus in the distribution of values of any linear measurement or index among the three species. The only character able to some extent to discriminate between the taxa is the shell size (see below).

The holotypes of the four Siberian species of Radix discussed in the paper (ZIN). A. Radix dolgini. B. R. kurejkae. C. R. gundrizeri. D. R. ulaganica. Scale bars: 2 mm.
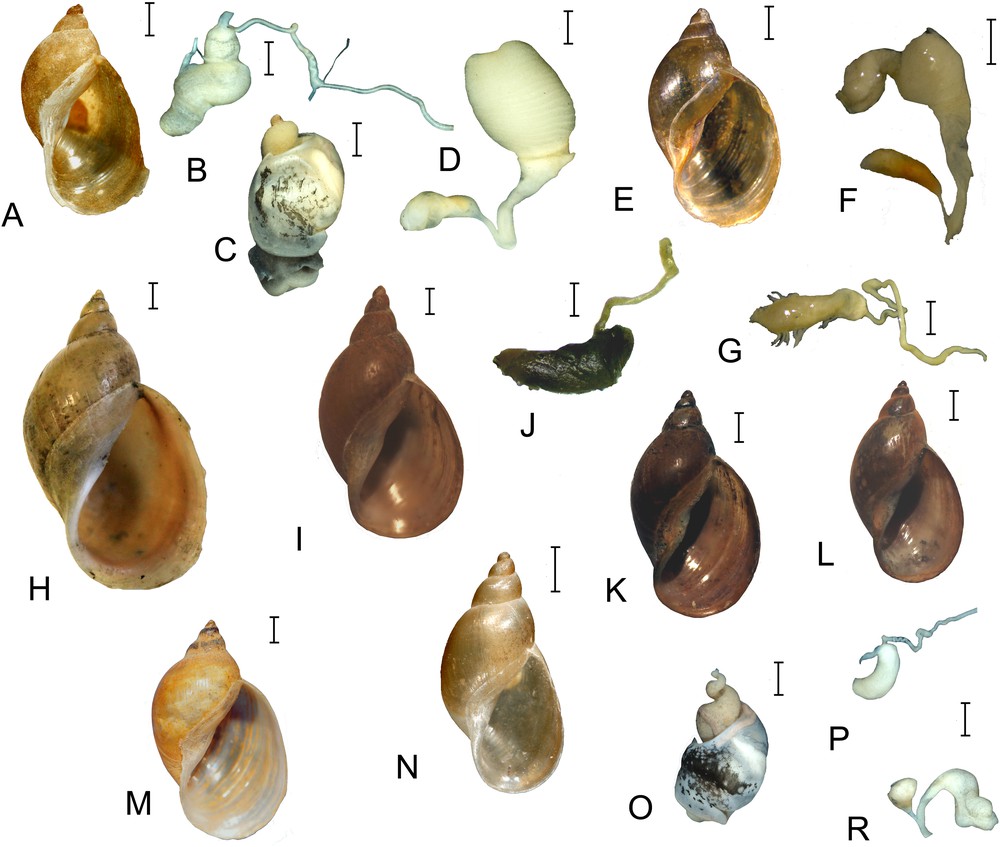
Shells and fragments of the reproductive system of Radix dolgini. A–D. Russia, Tomsk Region, a lake near Novoshumilovo village. E–G. Russia, Republic of Buryatia, Kironskiy Spring (RMBH). H. Russia, Altay Territory, a swamp in the floodplain of Kulunda River (MSAM = Lymnaea gundrizeri sensu Kruglov and Starobogatov). I and J. Russia, Tyumen Region, Lake Souskanovskoye (MSAM = L. gundrizeri sensu Kruglov and Starobogatov). K and L. Russia, Tyumen Region, Lake Souskanovskoye (MSAM = L. kurejkae sensu Kruglov and Starobogatov). M. Russia, Omsk Region, floodplain of Irtysh River near Kachesovo village (MSAM). N–R. Russia, Novosibirsk Region, an unnamed pool near Severnoye–Biaza Road (= L. ulaganica sensu Kruglov and Starobogatov). B, J, G, P – copulatory apparatuses; C, O – external view of soft bodies; D, F, R – female reproductive tracts. Scale bars: 1 mm (soft bodies and genitals), 2 mm (shells). A–C and N–R – photos made by P. Glöer. Masquer
Shells and fragments of the reproductive system of Radix dolgini. A–D. Russia, Tomsk Region, a lake near Novoshumilovo village. E–G. Russia, Republic of Buryatia, Kironskiy Spring (RMBH). H. Russia, Altay Territory, a swamp in the floodplain of Kulunda River ... Lire la suite
The PCA analysis of shell variation has shown that there is no reliable distinction between specimens of R. dolgini and R. ulaganica that fell into the same cloud of points in the multivariate space (Fig. 4). The specimens of R. gundrizeri, although, are separated from the R. dolgini–R. ulaganica cloud alongside the first PC only and have no relation to variations in the second PC. The analysis of the factor loadings (Table 3) says that the first PC is strongly correlated with all variables, and may thus be interpreted as the compound ‘size’ variable. The second PC is correlated with the number of whorls and the spire height, which are thereby factors related to the proportions of the shell. It means that the differences between R. gundrizeri and R. dolgini–R. ulaganica are nothing more than differences in absolute shell size, not in shell proportions that once have been used to substantiate the species status of the three taxa. The third PC is not strongly correlated with any variable and explains only 1.80% of the overall variation. We are unable to propose a biologically sound interpretation for PC3 and thus omit it from consideration.
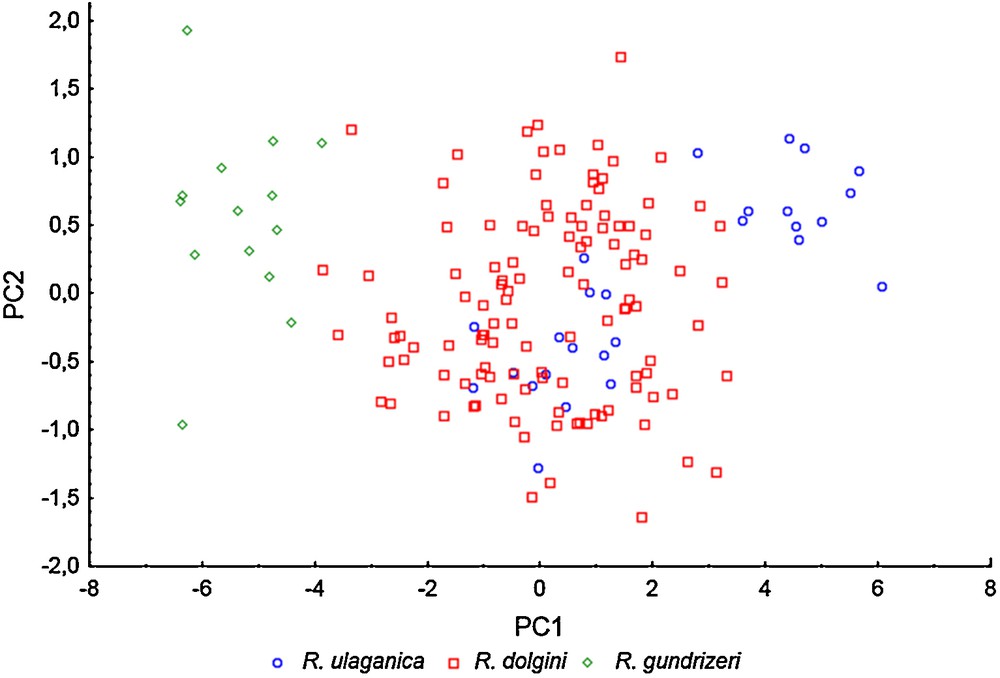
The principal component analysis (PCA) of shell variation in three Siberian species of Radix. PC1 explains 90.64% of the total variation; PC2 – 6.01%.
Factor loadings of traits in the principal component analysis (PCA) of shell variation in the Siberian endemic Radix species.
| Trait | PC1 | PC2 | PC3 |
| Number of whorls | –0.90 | 0.32 | –0.28 |
| Shell height | –0.99 | 0.02 | 0.05 |
| Shell width | –0.96 | –0.24 | 0.01 |
| Spire height | –0.88 | 0.42 | 0.21 |
| Body whorl height | –0.99 | –0.06 | 0.04 |
| Aperture height | –0.97 | –0.17 | –0.03 |
| Aperture width | –0.96 | –0.23 | 0.002 |
| % variation explained | 90.64 | 6.01 | 1.80 |
A similar result was obtained by means of another multivariate technique, CVA (not showed).
The structure of the reproductive system in R. dolgini, R. gundrizeri, and R. ulaganica is virtually identical. All these species are characterized by a relatively large praeputium and a very narrow penis sheath. In most studied samples, the lengths of the praeputium and of the penis sheath reflected in the ICA values are nearly equal, with the penis sheath being a little longer than the praeputium. The mean ICA values of most samples do not exceed 1.0 (Table 4). The only exception is a small series of four dissected specimens from the Kulunda River floodplain identified as R. gundrizeri. The snails of this sample possess very long praeputia that are about 1.5 times longer than their penis sheaths (see Table 1). However, this difference has not been reflected in the molecular trees (see Fig. 1).
ICA variation in the samples of R. dolgini and related species.
| Species, locality | ICAa |
| R. dolgini, Russia, Omsk Region, Ananyevskoye near Atak village (n = 21) |
|
| R. dolgini, Russia, Irkutsk Region, Kirensk Town, a lake in the floodplain of Kirenga River (n = 7) |
|
| R. gundrizeri, Russia, Altai Territory, a swamp in the floodplain of Kulunda River (n = 4) |
|
| R. gundrizeri, Russia, Tyumen’ Region, Lake Souskanovskoye (n = 5) |
|
| R. labiata, Germany, Saxony, a pond situated between Langenberg and Hammergut towns (n = 11) |
|
| R. labiata, Slovenia, bank of Radeščica River near Podhosta (n = 8) |
|
| R. labiata, Russia, Sverdlovsk Region, a roadside ditch near Khomutovka village (n = 4) |
|
| R. ulaganica, Russia, Novosibirsk Region, an unnamed pool near Severnoye–Biaza Road (n = 5) |
|
| R. ulaganica, Russia, Omsk Region, a forest swamp near Atak village (n = 5) |
|
a Above line: minimum and maximum values; below line: mean value ± standard deviation.
2.3 Habitat preferences
R. dolgini inhabits a wide variety of freshwater habitats, including lakes, river pools and oxbows, swamps and other wetlands, roadside ditches, streams and various temporary waterbodies (Table S2). In addition, we collected its specimens on an algae mat in a warm stream (T = 27.2–29.3 °С) that flows from Kironsky geothermal spring, Baikal Region.
3 Discussion
The results presented in the previous section show that there were five genetically distinct species of Radix (Peregriana) in our sample, four of them being earlier characterized morphologically and molecularly by Schniebs et al. [11,19], whereas the fifth species is endemic to Siberia, and its taxonomic distinctness is for the first time demonstrated here by means of molecular taxonomic methods.
Three nominal species of Siberian lymnaeids, R. dolgini, R. gundrizeri, and R. ulaganica, are most probably conspecific, and the oldest available name for their designation should be R. dolgini (Gundrizer and Starobogatov, 1979), whereas the two other species names become its junior synonyms. The clade formed by these species corresponds well to the “molecular species” approach [43] that may be formalized as follows. The molecular species is a group of individuals that constitute a separate diagnosable cluster on a cladogram [44]. However, the biological species are organisms, not simple sets of genes, and it is very desirable to invoke morphological and zoogeographical evidences in order to make our taxonomic opinion grounded on the genuinely integrative approach.
The morphological differences between R. dolgini, R. gundrizeri, and R. ulaganica are not prominent enough to be viewed as qualitative. All three species have a similar shell appearance, almost identical structure of the copulative apparatus, whereas all differences proposed to distinguish the three taxa are restricted to slight differences in proportions of the shell and the copulative apparatus [8,26]. However, it is well known that shell proportions as well as proportions of the copulatory organ in lymnaeids are prone to intraspecific variation [11,27,45–48], and their taxonomic significance is limited. The results of the PCA analysis of variation presented above support the hypothesis that the three species belong to the same morphologically defined entity, whereas the differences among them highlighted by Starobogatov et al. [26] and Kruglov [8] may disappear when large samples of snails are analysed. At least two species, R. dolgini and R. ulaganica, proved to be indistinguishable by means of two methods of multivariate statistics, PCA and CVA (see Fig. 4). The specimens of R. gundrizeri are somewhat different, being characterized by a larger absolute size of their shells, but the size differences as such are hardly enough to substantiate the species distinctness of R. gundrizeri, especially under the full absence of molecular support. It should be noted that the absolute shell size in freshwater molluscs is a plastic value effected by a range of environmental factors, including ambient temperature, predator press, infestation by parasites and so on [48–50]. Its taxonomic significance is thought to be negligible.
A question of validity of the fourth Siberian species, R. kurejkae, is a bit more difficult. We had no fixed specimens of this species so as to include it in our genetic analysis, and thus the reality of R. kurejkae has formally not been tested by the molecular taxonomic approach. However, there are at least two indirect evidences supporting its conspecifity with R. dolgini. First, the type series of both species, R. dolgini and R. kurejkae, were collected in the same locality (see Materials and methods). The type series of R. dolgini includes 13 specimens, whereas R. kurejkae was described on the basis of merely six individuals. The statistical significance of differences between syntopic specimens of the two species was not assessed by their authors [25], and it is impossible to reject a hypothesis that these differences may be explained by intrapopulation variation. Second, the conchological differences between R. dolgini and R. kurejkae reported in the Russian literature [8,25,26] are very slight. For example, Kruglov [8] proposed to discriminate between the two species by means of the ratio between the shell height (SH) and the shell width (SW). This ratio in R. kurejkae is less than 1.55, whereas in R. dolgini it exceeds 1.60 [8]. Having measured 117 shells from seven localities in Western Siberia, we found that it was quite impossible to divide this sample into two distinct subsamples that would correspond to the two alleged species. The variation of the SH/SW ratio is continuous, and no hiatus may be observed (Fig. 5). A portion of individuals have shell proportions intermediate between R. dolgini and R. kurejkae, i.e. their SH/SW ratio lie between 1.55 and 1.60 (see Fig. 5).
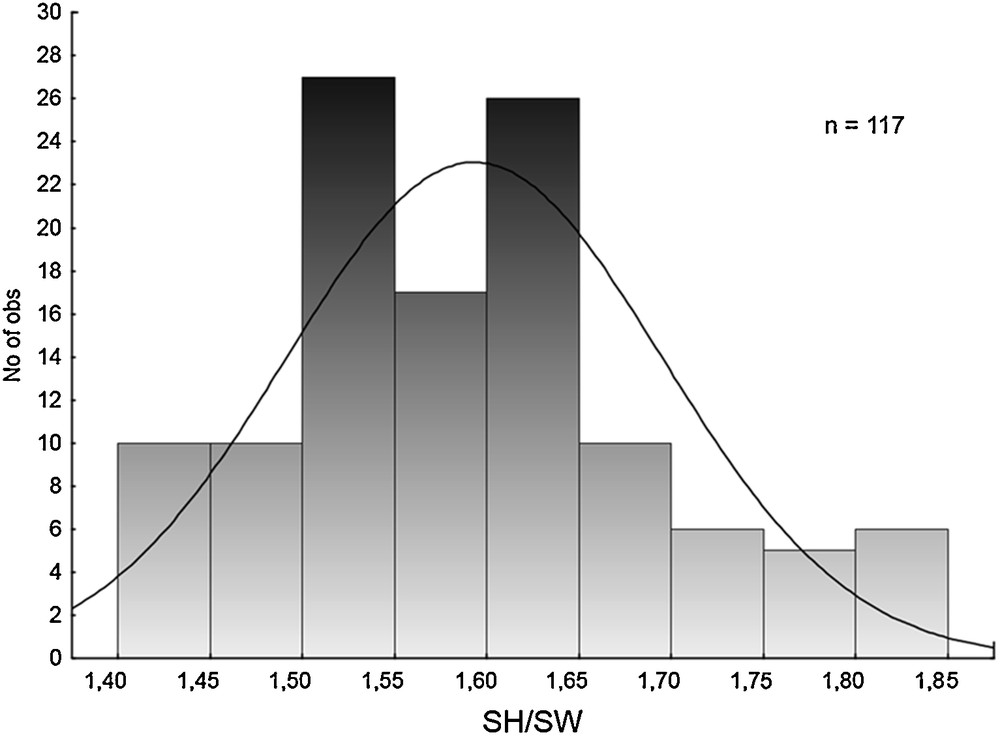
Variation of the SH/SW ratio in a sample of Radix dolgini s. lato from seven localities situated in Western Siberia.
All these considerations lead us to conclusion that R. kurejkae is identical to R. dolgini and should be treated as its junior synonym. The morphology of the type specimens (see Fig. 2) does not contradict this conclusion.
From the zoogeographical point of view, R. dolgini has a distinct distribution range that covers almost all Western Siberia and southern parts of Eastern Siberia (Fig. 6). A single finding of R. kurejkae is known from Lake Nogoon, Western Mongolia, a location situated close to the Russian part of Altay Mts. (ZIN collection). In Western Siberia, the range of R. dolgini overlaps slightly with the range of R. labiata along the southwestern part of the Ob’-Irtysh River system (see Fig. 6), but it seems that the former species is a Siberian vicariant of the latter. Their range overlap in Western Siberia may have been caused by secondary intergradation.
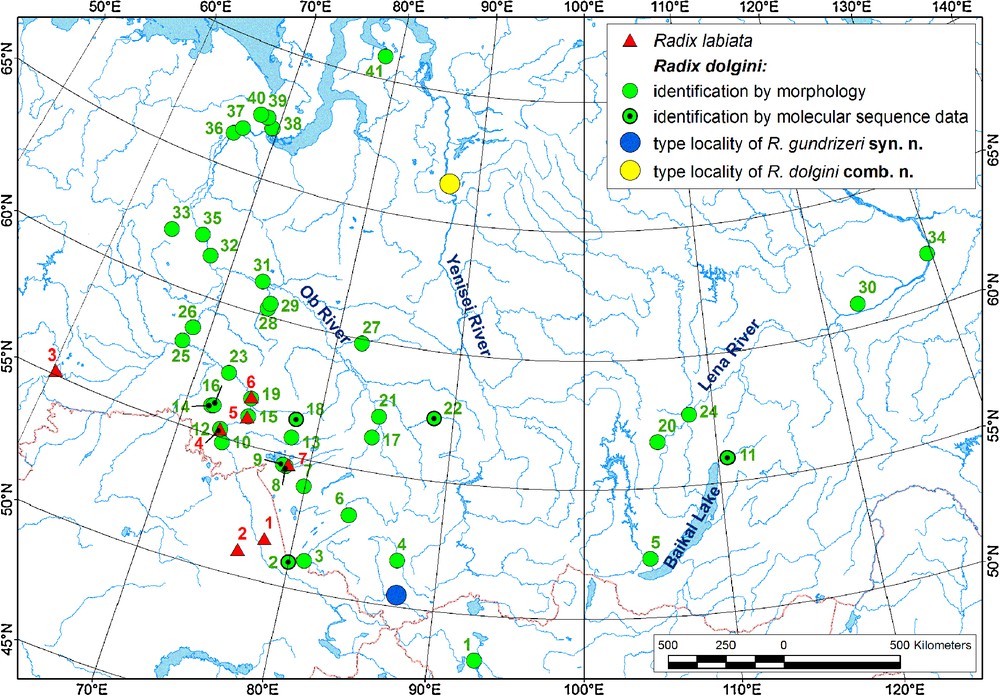
Records of Radix dolgini and R. labiata in Siberia. 1 – R. labiata; 2–3 – R. dolgini (2 – identification by morphology; 3 – identification by morphology and molecular sequence data); 4 – type locality of R. gundrizeri syn. n. and R. ulaganica syn. n.; 5 – type locality of R. dolgini and R. kurejkae syn. n. The locality data are shown in Tables S2 and S3; the colour numbers of the species localities on the map correspond to the numbers in these tables (red numbers – R. labiata; green numbers – R. dolgini; records situated close to each other are concatenated). Masquer
Records of Radix dolgini and R. labiata in Siberia. 1 – R. labiata; 2–3 – R. dolgini (2 – identification by morphology; 3 – identification by morphology and molecular sequence data); 4 – type locality of R. gundrizeri syn. n. and R. ulaganica syn. ... Lire la suite
Taking into account that some species of aquatic continental animals having a Siberian origin later migrated to Northern Europe [51], it is reasonable to await that R. dolgini may be found in the northeastern part of Eastern Europe, for example, in the Pechora River basin.
In accordance with a mean divergence rate under the HKY + I + G model as 3.14% My−1 [52] and a mean distance between R. dolgini and R. labiata COI haplotype groups of 10.2 ± 1.6%, we can assume that these species were derived from a common ancestor in the Late Pliocene, approximately 3.25 My ago. The mid-Pliocene was a global warm episode, interrupted at ∼ 3.3 Ma by a globally recognizable cooling event corresponding to the marine isotope stage (MIS) M2 [53–55]. Based on oxygen isotopic data inferred from benthic foraminifera combined with ostracod magnesium/calcium (Mg/Ca)-based bottom-water temperatures, Dwyer and Chandler [56] reported a low stand of sea level of approximately 65 m below present at MIS M2, which followed by strong fluctuation of 20–30 m above and below a mean value similar to the present-day sea level. In general, paleogeographic data for the MIS M2 stage are very poor, and detailed reconstructions of a coastal line and of the ice sheet contours are absent. However, Dolan et al. [55] noted that the modelling results are not able to discount the possibility of significant ice masses in the Northern Hemisphere during the M2 event, in a manner consistent with a global sea level fall of 40 to 60 m.
The most popular scenario for the formation of the Siberian freshwater malacofauna [57,58] assumes that the core of the fauna is constituted by cold adapted species of European origin that invaded Siberia during the Pliocene and the Pleistocene. The existence of glacial refugia and diversification centres of aquatic fauna in Siberia have been also proposed [8,57,59]. It was hypothesized that R. dolgini and related species arose in the Western Siberian Pliocene diversification centre situated somewhere in the southern part of Western Siberia [8,59]. Our results are, in general, compatible with such a scenario, but we do not focus on a main direction of dispersal events in an ancestor or in the modern descendants from Europe to Siberia or vice versa, because no evidence for such a discussion is accessible. In contrast, our data constitute a new molecular evidence for a hypothetical physical barrier between Europe and Siberia during this stage, which likely contributes to a separation of the paleo-range of the common ancestor of R. labiata and R. dolgini on two isolated areas. It is known that a fossil species, which had a shell morphology very similar to that of R. labiata, existed in Europe as early as in the Pliocene [60] and may be considered as a possible common ancestor of this species pair. Our assumptions are in accordance with published data for other freshwater hydrobionts, i.e. the similar speciation models were suggested for some fish taxa. For example, Bohlen et al. [61] assumed a continuous distribution of a common ancestor of the recent bitterlings (Rhodeus spp.) from Europe through Siberia until East Asia during Pliocene, a possible extirpation of bitterlings from Siberia in the Late Pliocene or the Early Pleistocene and the subsequent separation of the Eastern Asian population from the European one.
A possible existence of an extended dispersal barrier for freshwater hydrobionts between Europe and Siberia in the Late Pliocene seems to be very important in a biogeographical sense. It is known that the Western Siberian Seaway (Turgai Strait), a connection of the Peri-Tethys with the Arctic Ocean, which has existed during the Palaeocene and the Eocene, played a great role in the freshwater biogeography patterns of the Palaearctic Region [62,63]. However, the probability of existence of a more recent barrier on the territory of Western Siberia has not been previously discussed in detail, although it could have a significant influence on the origin of freshwater faunas of Eastern Europe and Siberia.
The situation, when two snail species, R. dolgini and R. labiata, form a pair of vicariant species with European and Siberian distribution is a new addition to a long list of similar pairs of continental animals, both terrestrial and aquatic, known to demonstrate the same pattern of vicariance. Such examples are known in freshwater amphipods [64], amphibians [65], fish [66], and mammals [67]. In lymnaeid snails, a somewhat resembling situation was found in the species Lymnaea stagnalis (L.) s. lato, with two intraspecific phylogroups revealed by genetic methods [68,69]. Mezhzherin et al. [69] consider them as representing the geographically defined allospecies, the “western” and the “eastern” ones.
Ecologically, R. dolgini may be defined as a typical dweller of temporary or semi-temporary habitats such as wetlands, swampy lakes, pools and like (see Supplementary material 2). It often lives in the floodplain waterbodies. In this respect, R. dolgini resembles its sister species R. labiata, which is characterized by similar ecological preferences [6,20].
Below, the re-description of the species R. dolgini is given.
Radix (Peregriana) dolgini (Gundrizer and Starobogatov, 1979)
= Lymnaea dolgini Gundrizer and Starobogatov, 1979: 1132, fig. 1(2), 2 (2).
= Lymnaea kurejkae Gundrizer and Starobogatov, 1979: 1131, fig. 1(1), 2(1).
= Lymnaea gundrizeri Kruglov and Starobogatov, 1983: 139.
= Lymnaea ulaganica Kruglov and Starobogatov, 1983: 141.
Shell ovate-conical, slender (as compared with most other species of Peregriana), with prominent cone-shaped spire, flattened whorls and almost straight tangent-line. The shell height reaches 20–22 mm (the largest specimen we have seen was 22.5 mm high), but usually does not exceed 20 mm. Aperture drop-like, with narrow angle in its upper part. The shell is characterized by a rather wide and thin columellar lip, white-coloured or almost transparent, which covers the umbilicus entirely. The praeputium (PP) is sack-like, light pigmented, the penis sheath (PS) is very narrow as compared to PP, the lengths of PP and PS are nearly equal. The spermathecal duct is short but always prominent.
R. dolgini differs unambiguously from R. ampla and R. balthica by its shell shape, but confusion with R. labiata and R. lagotis is possible. Since there are no significant differences among these species in proportions of their copulatory apparatuses, the genetic data and conchological characters remain the most useful tools for their discrimination.
As compared with R. dolgini, R. labiata is characterized by a typically smaller shell, whose height is usually well under 20 mm and in most instances does not exceed 15 mm (Fig. 7). The whorls of the spire in R. labiata are much more inflated than in R. dolgini, and the columellar lip is apparently narrower (Fig. 8). However, there are no significant differences between these two species in their ICA values (see Table 3).
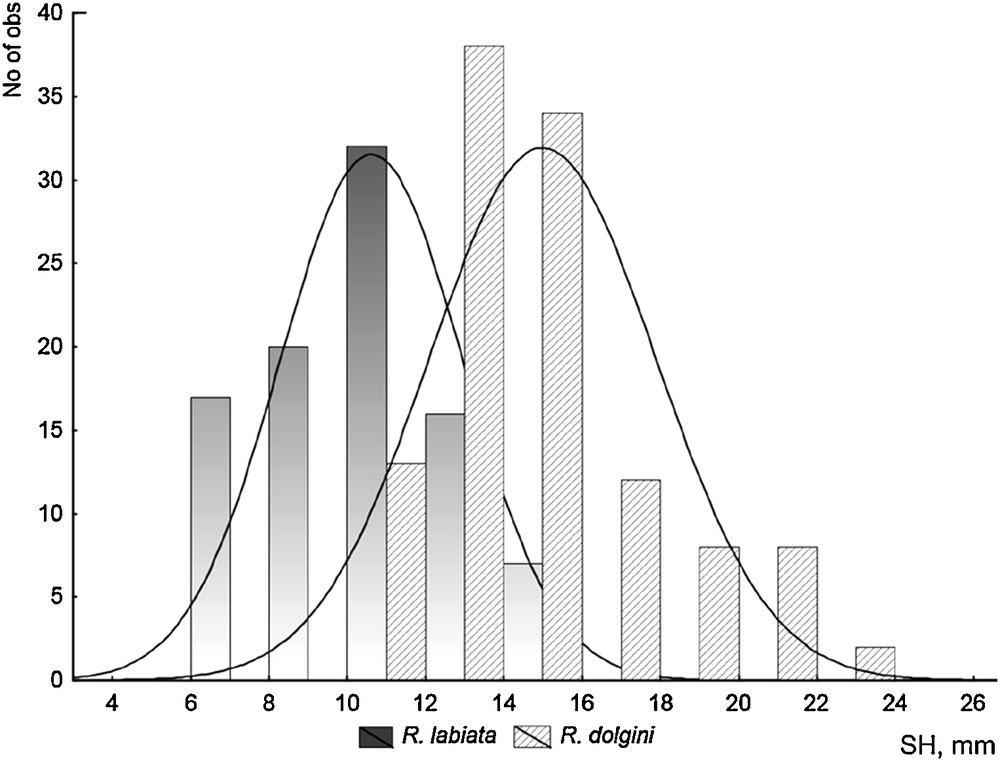
Shell height distribution in the samples of R. dolgini (n = 117) and R. labiata (n = 92).

Shells of R. dolgini (A, B), R. labiata (C, D), and R. lagotis (E, F). A, B–Taken from Fig. 3; C. Russia, Omsk Region, Pyataya rechka River near Tara Town (MSAM). D. Austria, Carinthia, Kreutzberg (ZMB). E. Russia, Baikal region, Mysovaya, a lake near beacon (LIN). F. Denmark, Amager Fælled, Copenhagen (ZMUC). Scale bars: 2 mm.
The shell of R. lagotis is of similar size as compared to the shell of R. dolgini, but the former species has narrower spire and a bit more inflated body whorl. The tangent-line of the R. lagotis shell is concave, whereas in R. dolgini it is almost straight. The columellar lip in R. lagotis is not wide, and the umbilicus is visible (see Fig. 8F).
One must remember, however, that all conchological traits proposed above to discriminate between R. dolgini and allied species are prone to intra- and interpopulation variation, and as such may sometimes be misleading. The most effective tool for unequivocal species identification of the Radix species is the molecular taxonomic analysis.
The analysis of a habitat allocation of the R. dolgini populations (Table S2) reveals that it is a rather eurytopic species like the majority of other Radix spp. and lymnaeids in general [8]. However, the record of its population in the Kironsky geothermal spring, Baikal Region, has a considerable interest because it is the third Radix species, an inhabitation of which in geothermal habitats was confirmed using the integrative taxonomic approach based on both morphological and molecular data. In the Khodutka geothermal system on Kamchatka, Bolotov et al. [70] found R. auricularia, different morphological forms of which were previously considered as two local species described as endemic to this site. In addition, Quintela et al. [71] recorded R. balthica populations from some Icelandic geothermal springs. According to these data, various widespread Radix species could successfully colonize local geothermal ecosystems across the Northern Palaearctic Region. There are several additional works, which are reported occurrences of various Radix spp. from different geothermal locations across Eastern Siberia (e.g., [72–74]). Unfortunately, the actual taxonomic affinities of these specimens are uncertain, because all of these reports are based exclusively on the morphological identification of snails.
4 Conclusions
Though more than twenty nominal species of the genus Radix have been registered in the waterbodies of Siberia [3,4,8,20], most of them are delineated on the basis of slight differences in shell growth pattern and/or in the proportions of the copulative organ [8]. The integrative taxonomic approach used in this study has revealed that morphology-based species delimitation in the lymnaeid snails may lead to an overestimation of their true species richness. We revealed that the rate of synonymy might be close to 4:1 (four morphospecies appeared to represent a single taxon of the species rank), at least in the genus Radix. To date, six species of this genus (including R. dolgini) in the fauna of Europe and Siberia have sustained the “molecular” ordeal and proved to be “good” species from morphological, genetic, ecological, and zoogeographical points of view. Much more nominal species of Radix and other lymnaeid genera in Northern Asia still await their verification by means of integrative taxonomic scrutiny.
Acknowledgements
We thank T.Ya. Sutnikova (LIN, Irkutsk), L.L. Yarokhnovich and P.V. Kiyashko (ZIN, Saint-Petersburg), T. Schiøtte (ZMUC, Copenhagen), and C. Zorn (ZMB, Berlin) for their help during work with museum collections. Also, we have to express our sincere gratitude to Peter Glöer (Hetlingen, Germany) for excellent photos of some specimens from Siberia. The two reviewers (Manuel Lopez-Lima and an unknown one) are acknowledged for their constructive criticism that helped us to improve the original text. This study was partly supported by grants from the President of Russia (No. MD-6465.2014.5 and МК-4735.2015.4), the Federal Agency for Scientific Organizations (No. 0410-2014-0028), the Russian Ministry of Education and Science (project No. 6.1957.2014/К), the Ural Branch of the Russian Academy of Sciences (Nos. 15-12-5-3 and 15-2-5-7), and the Russian Foundation for Basic Research (Nos. 14-04-98801, 14-04-01236, 14-04-31657_mol_a, and 15-04-05638). We would like to express our thanks to Prof. Dr Uwe Fritz (SNSD) for financial support for sequences done by K.S.


