1. Introduction
A universal characteristic among all living organisms, from unicellular to multicellular systems, is the ability to undergo cell division, a critical process that ensures survival and propagation. Cell division starts by the duplication of the genetic material and organelles, followed by their segregation in two daughter cells during cytokinesis.
Cytokinesis is the process that takes place at the end of cell division and that starts by the invagination of cell membrane between the two daughter cells. This invagination is driven by the constriction of an actomyosin ring at the cell cortex. Simultaneously, the mitotic spindle is remodeled into an electron-dense structure called the midbody. Abscission then takes place on one side of the midbody with the cleavage of the membrane and the physical separation of the two daughter cells [1, 2]. Most eukaryotic cells complete abscission. In contrast, the development of specific cell types relies on incomplete cytokinesis, where cells remain connected by stable intercellular bridges called ring canals to form cysts. The maintenance of cell connectivity is an evolutionary conserved feature of germ cells and was also described in some somatic tissues [3, 4, 5, 6, 7, 8, 9]. In the male germline, the progression of sperm development leads to the formation of haploid cells in which the X and Y sexual chromosomes are segregated in different cells. However, all cells require gene products from both X and Y chromosomes for their correct differentiation. In this context, arrested cytokinesis allows X and Y chromosome gene products to be shared between all cells, although they are genetically different. Therefore, incomplete cytokinesis in the male germline complements the genetic content of the future gametes. Most female germlines also develop as cysts. In meroistic ovaries, only a subset of germ cells acquire an oocyte fate, while their siblings become nurse cells. In such cysts, nurse cells transfer material to the future oocyte through the ring canals to allow its massive growth and differentiation. Ultimately, nurse cells are eliminated by apoptosis. This nursing mechanism has been extensively documented in Drosophila female germline, and was more recently described in mouse ovaries as well. This nursing mechanism is essential in both species for proper oocyte development as the oocyte is mostly transcriptionally silent [10, 3]. In mice and fly, mutations affecting germline cyst formation lead to animal sterility, highlighting the importance of developmentally regulated incomplete abscission [11, 12].
Here, we will briefly review the molecular mechanisms of complete abscission described in most animal cells and how these molecular mechanisms are modulated to delay abscission. We will then focus on abscission in Drosophila germline cells, and the recent discovery of a key enzyme, Usp8, which is both necessary and sufficient to delay abscission in Drosophila germline cells. The results about Usp8 presented here were originally published in Science [12] and selected by the Académie des sciences.
2. Molecular mechanisms of abscission
ESCRT-III (for Endosomal Sorting Complex Required for Transport-III) are small proteins, able to polymerize into helical filaments thought to be the force driving membrane scission. The ESCRT machinery was initially identified in Saccharomyces cerevisiae as a group of proteins working sequentially to sort membrane receptors into the vacuole [13, 14, 15]. Three complexes, ESCRT-I, II and III allow the transfer of ubiquitinated receptors into the lumen of the endosomes through the formation of the intra-luminal vesicles [16, 17]. In parallel to ESCRT-I and II, Alix protein also recruits ESCRT-III on endosomes. ESCRT-III proteins cycle between an inactive closed cytoplasmic state to an open active conformation, able to polymerize and interact with MIT-domain containing proteins. Vps4 is an MIT-domain AAA-ATPase that promotes the dynamic turnover of ESCRT-III filaments required for membrane scission [18, 19, 20, 21]. The ESCRT machinery described for the biogenesis of MVB (multi vesicular bodies) in yeast is conserved in animal cells, where it is used in many additional membrane-remodeling processes such as virus budding, neuron pruning, nuclear envelope reformation, exosomes release, or cytokinetic abscission. In human cells, CEP55 is localized at the midbody and recruits Alix and Tsg101, both of which then mediates the recruitment of ESCRT-III. A secondary pool of ESCRT-III appears on the side of the midbody, at the site where membrane scission takes place. Depletion of individual mammalian ESCRT-III proteins, or of the proteins in charge of their localization delays abscission in animal cells [22, 23, 24, 25, 26, 27, 28]. During abscission, ESCRT-III turnover in the filaments is regulated by Vps4 [29]. Each ESCRT-III has the intrinsic ability to form bent filaments, with a specific curvature, which allows them to form either flat spirals or helices [30]. Each ESCRT-III proteins has a selective affinity for specific membrane curvature and is sequentially recruited on membranes to promote membrane deformation followed by scission in vitro [31, 32]. However, how ESCRT-III proteins are organized in vivo within the cytokinetic bridge is still not fully understood [33].
In mammalian cells, abscission takes place in G1 phase of the next cell cycle before the start of DNA replication. However, the timing and duration of abscission can be coordinated with other cellular events. For example, in case of spindle defects or lagging chromosomes, abscission is delayed by Aurora B-dependent checkpoint [34]. In addition, this checkpoint is also activated when nuclear pores are not properly formed or when strong forces are applied on the bridge [35, 36, 37, 38]. Several targets of Aurora B have been described in this process, such as the ESCRT-III protein CHMP4C [39], as well as ANCHR [40] or the UKL3 kinase [41]. All of them localize to the midbody and have the ability to delay abscission. In the cytoplasm, abscission checkpoint bodies containing Aurora B, CHMP4C and Alix also delay abscission by preventing ESCRT-III recruitment at the midbody [42].
3. Abscission in Drosophila germline cells
Apart from being induced by cellular defects, delayed cytokinesis is also observed in many developmental contexts, and is a conserved feature of germline cells [9]. Because of the powerful genetics of Drosophila and its well-described oogenesis, Drosophila germline is an established model to understand incomplete cytokinesis. In adult females, germline stem cells (GSC) divide every day at the tip of a specialized structure called the germarium. These divisions are complete, even though abscission in these cells takes place late, in G2 of the next cell cycle [27, 43, 44]. The division of the GSC is asymmetric and generates a new GSC and a cystoblast (CB). The differentiation program of the cystoblast involves four rounds of synchronous divisions with incomplete cytokinesis and leads to the formation of a 16-cell cyst (Figure 1A). Once the cyst is formed, one cell is specified as the oocyte and the 15 others become nurse cells. These cysts then mature as egg chambers (Figure 1A). The molecular mechanisms that regulate abscission in the GSC have started to be elucidated, and the involvement of the ESCRT machinery was demonstrated. In fly, Alix is recruited directly by Pavarotti/MKLP1 [45]. Although complete loss of Alix is viable, females are sterile and their GSCs exhibit a strong abscission delay [28]. Shrub is the single and essential Drosophila homolog of CHMP4; removing one copy of the Shrub gene is sufficient to induce a strong GSC abscission delay [27]. As in mammalian cells, Aurora B activity delays abscission in GSCs. Abscission delay of the GSCs can be visualized by the formation of “stem cysts”, made of sister cells having a GSC identity (“stem-”) but which remain connected by ring canals (“-cyst”) [46]. In these stem-cysts, the fusome, an ER derived organelle specific to the germline, passes through the ring canals as in wild-type cysts (Figure 1B). Eventually, abscission occurs and releases a cystoblast made of two cells instead of one cell. Then, this two-cell cystoblast enters the differentiation program and goes through four incomplete divisions leading to the formation of 32-cell cysts instead of 16 (Figure 1A,B). Conversely, in mutant females for a hypomorphic allele of Aurora B (low Aurora B activity), abscission occurs ectopically in germline cysts instead of remaining incomplete [46]. The outcome of these ectopic abscissions is the formation of germline cysts made of 8 cells or less (Figure 1C).
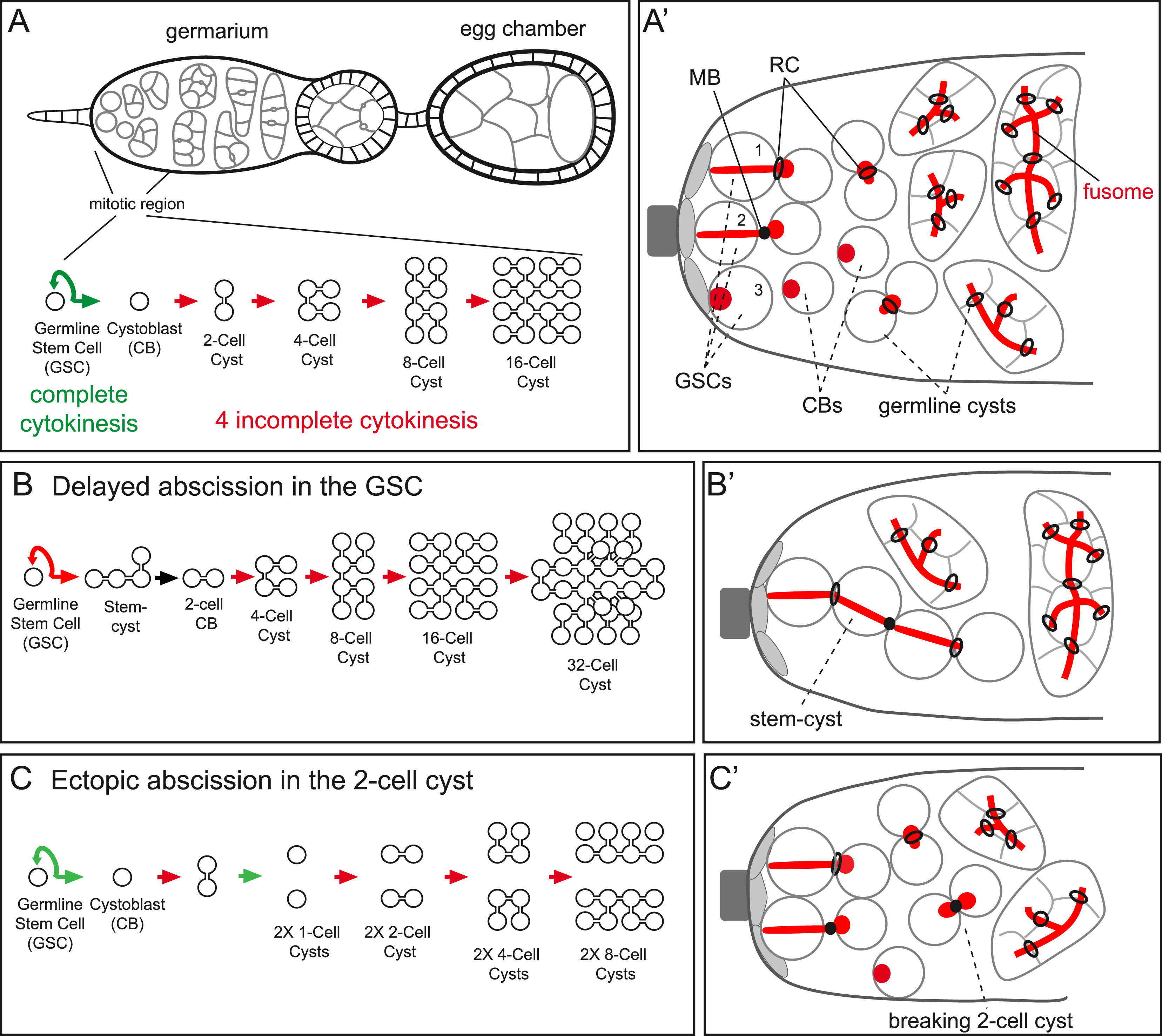
(A) Top: female ovariole. Bottom: cell lineage of the germline stem cell (GSC). (A′) Mitotic region of the germarium. At the end of mitosis, GSCs are connected to their daughter CB through a ring canal (RC) (GSC #1) and later by a midbody (MB) (GSC #2). After complete cytokinesis, GSCs and CBs are fully separated (GSC #3). The fusome (red) links the GSC and the CB before abscission, and also passes through all RCs of germline cysts. Cap cells (light grey); terminal filament cells (dark grey). (B) Cell lineage of a GSC with delayed abscission. (B′) Mitotic region of a germarium when the GSC exhibit delayed abscission: stem-cysts are detected by the fusome passing from an anterior GSC to several cells. (C) Cell lineage of a cyst with ectopic abscission at the 2-cell stage. (C′) Mitotic region of a germarium when a 2-cell cyst exhibit ectopic abscission: a MB is detected between the two sister cells. (A,A′) were also presented in [12]; (B,B′), (C,C′) are original schemes. Masquer
(A) Top: female ovariole. Bottom: cell lineage of the germline stem cell (GSC). (A′) Mitotic region of the germarium. At the end of mitosis, GSCs are connected to their daughter CB through a ring canal (RC) (GSC #1) and later ... Lire la suite
To gain insights into the molecular mechanisms that regulate the incomplete abscission of the germline cysts, we performed a genetic screen. We knocked down potential ESCRT-III interactors in Drosophila germline cells, and analyzed the numbers of cells in each egg chamber. With this simple assay, we identified the gene Usp8 as necessary for incomplete abscission: cysts lacking Usp8 undergo complete abscission, and egg chambers of 8 cells or less are formed as a consequence (Figure 2A,B). On the other hand, over-expression of Usp8 is sufficient to delay abscission in GSCs, as revealed by the appearance of stem-cysts and the formation of 32-cell egg chambers (Figure 2C). Over-expression of Usp8 is also sufficient to block abscission in germline cells that are unable to differentiate into cysts (Figure 2D,E). Therefore, we concluded that Usp8 is necessary and sufficient to promote incomplete cytokinesis in Drosophila germline cells.
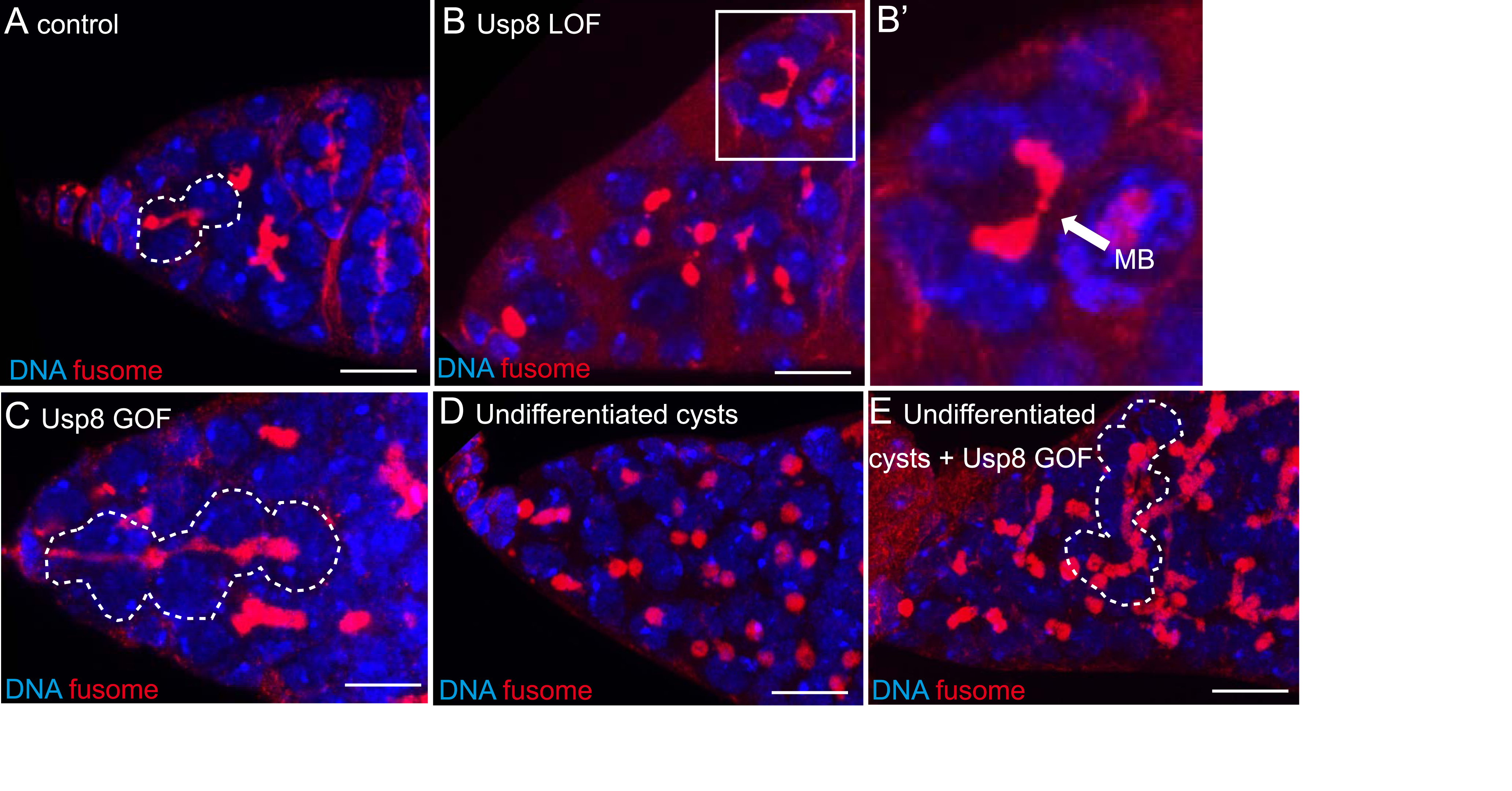
Confocal images of fixed germaria of control female (A), female with Usp8 loss of function (LOF) (B,B′), female overexpressing Usp8 (Gain of function, GOF) (C), females with cysts unable to differenciate (bam mutant, (D)), and females with cysts unable to differenciate but overexpressing Usp8 (E). (B′) is a close-up images of the cysts framed in (B). Dotted lines surround a GSC/CB pair (A), a stem cyst (C) and a branched fusome indicating incomplete abscission (E). The white arrow indicates the ectopic midbody (MB) of a breaking cyst showing complete abcission (B′). Scale bar: 10 μm. (A–C) were also presented in [12], (D,E) are original results. Masquer
Confocal images of fixed germaria of control female (A), female with Usp8 loss of function (LOF) (B,B′), female overexpressing Usp8 (Gain of function, GOF) (C), females with cysts unable to differenciate (bam mutant, (D)), and females with cysts unable ... Lire la suite
We next investigated the molecular mechanism underlying the ability of Usp8 to block cytokinesis in germline cysts. We tagged endogenous Usp8 with GFP and found that it localizes at ring canals in both GSCs and germline cysts (Figure 3A,B). Usp8 is a deubiquitinating enzyme that removes ubiquitin moiety on its substrates. On the one hand, biochemical experiments showed that Shrub, CHMP2B and CHMP1, three ESCRT-III proteins, are all ubiquitinated in Drosophila cells [12]. On the other hand, we showed that Usp8 is able to deubiquitinate Shrub and CHMP2B (Figure 3C). In wild type germline cysts, neither Shrub nor CHMP2B are enriched at the ring canals linking the sister cells. However, both proteins become enriched in the absence of Usp8 (Figure 3D–F and [12]). These results indicate that deubiquitination of ESCRT-III by Usp8 is necessary to block their enrichment at ring canals. To test if this ectopic ESCRT-III localization is responsible for the complete abscission observed in Usp8 mutant germline cysts, we reduced Shrub or CHMP2B levels in Usp8 depleted cysts. In this double-mutant context, we found less ectopic abscissions in cysts, and egg chambers were formed with more cells at later stages (Figure 3G,H). Altogether, we concluded that Usp8 is required in cysts to deubiquitinate ESCRT-III, to block their recruitment at the ring canals and subsequent abscission. This result also raised the question of the importance of ubiquitination of ESCRT-III during complete abscission. To address this issue, we analyzed the ability of a non-ubiquitinatable form of Shrub (Shrub-KR) to rescue the abscission delay phenotype induced by a loss of Shrub in GSCs. We found that unlike wild type Shrub, non-ubiquitinatable Shrub is unable to localize to the ring canal and to promote abscission (Figure 3I–K). This result indicates that ubiquitination of Shrub is necessary for its proper localization and function during GSC abscission.
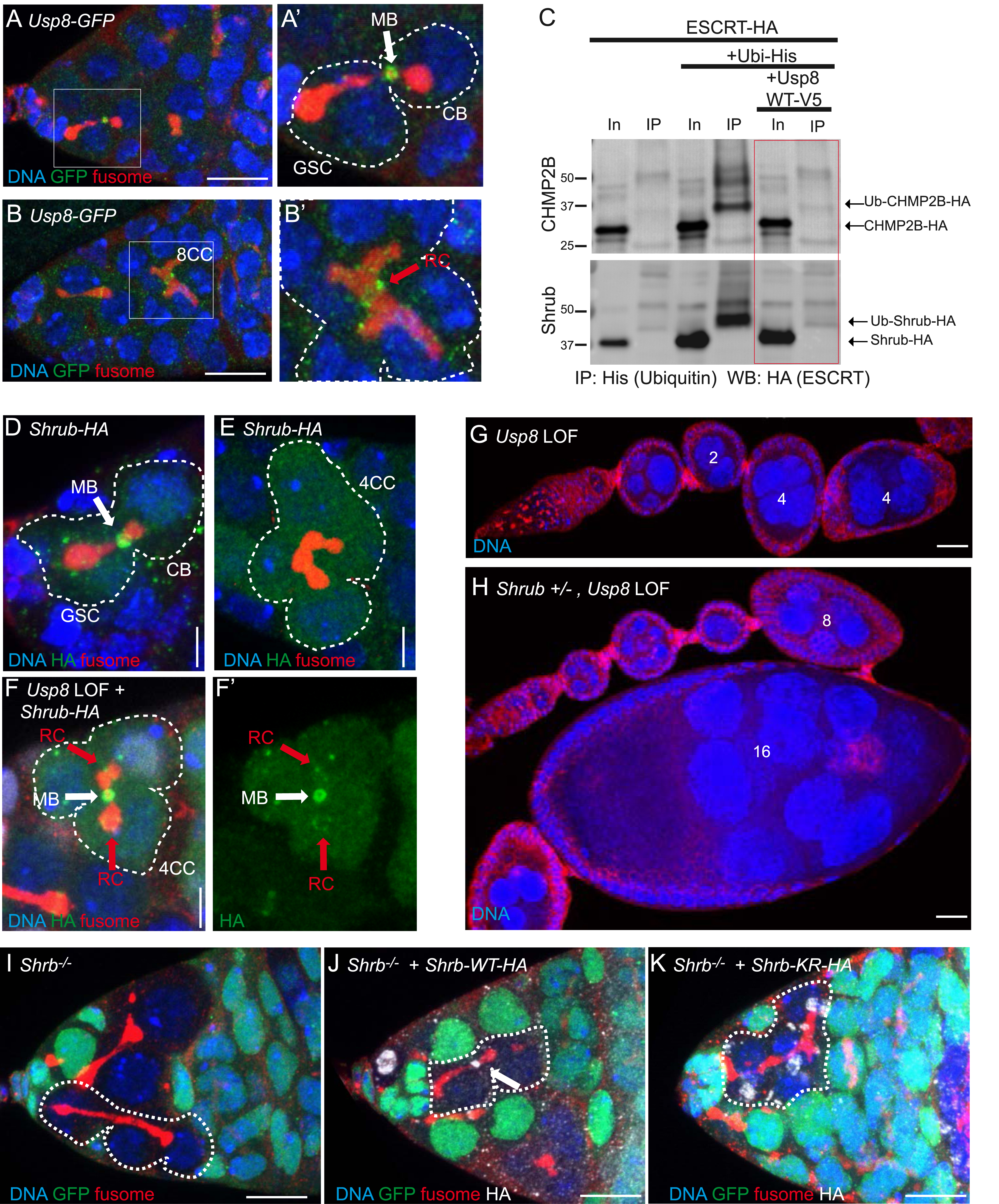
(A,B) Confocal images of germaria expressing GFP-tagged Usp8 from the endogenous locus. (A′) and (B′) are close-up of the cells framed in (A) and (B). Dotted lines surround a GSC and its daughter CB (A′), and a germline cyst (B′). The white arrow indicates the MB between the GSC and the CB in (A′), and the red arrow indicates the central ring canal in (B′). (C) Immunoblots of Input (In) or Immunoprecipitated (IP) ubiquitinated proteins from S2 cells transfected with ESCRT-HA, His-tagged Ubiquitin, and V5-tagged Usp8. Membranes were blotted with 𝛼-HA antibody, to reveal ubiquitinated ESCRT-III. Usp8 transfection is sufficient to abolish CHMP2B and Shrub ubiquitination (red frame). (D,E) Confocal images of fixed germaria of females expressing Shrub-HA. A GSC/CB pair (D) and a 4-cell cyst (4CC, (E)) are surrounded by dotted lines. The white arrow in (D) indicates the MB between the GSC and the CB. Shrub-HA is not detected at the 4CC RC. Scale bar: 5 μm. (F) Confocal images of a fixed germarium of a female expressing Shrub-HA and lacking Usp8 (Loss of function, LOF). The white arrow indicates the ectopic MB of the breaking 4CC, the red arrows indicate ectopic Shrub-HA at the RCs of the mutant 4CC. Scale bar: 5 μm. (G,H) Confocal images of a fixed ovariole from a female lacking Usp8 (Usp8 LOF) in an otherwise wild-type (G) or shrb heterozygous background (H). The number of nuclei of some egg chambers are indicated. Scale bar: 20 μm. (I–K) Confocal images of fixed germaria with shrb−/− germline cells (GFP negative) expressing Shrb-WT-HA (J), Shrb-KR-HA (K) or no transgene (I). Dotted lines surround a GSC/CB pair (J) or stem-cysts (I,K). White arrow indicates the MB decorated by Shrb-WT-HA. Shrb-KR-HA does not localize at the RC and is unable to rescue the stem-cyst. Scale bar: 10 μm. These results were originally presented in [12]. Masquer
(A,B) Confocal images of germaria expressing GFP-tagged Usp8 from the endogenous locus. (A′) and (B′) are close-up of the cells framed in (A) and (B). Dotted lines surround a GSC and its daughter CB (A′), and a germline cyst (B′). ... Lire la suite
Our data showed that Usp8 blocks the accumulation of ESCRT-III at the ring canals of germline cysts. However, ESCRT-III proteins do accumulate at the GSC ring canal despite the presence of Usp8. So, the presence or absence of Usp8 alone cannot explain the difference between complete and incomplete cytokinesis. We hypothesized that the difference of cell cycle duration between GSCs and germline cysts could explain this apparent contradiction. Indeed, each GSC divides only every 20 h, its abscission is very long and lasts until the G2 phase of the next cell cycle. By contrast, the four cycles leading to the formation of a 16-cell cyst last 24 h, that is, 6 h for each cycle. We analyzed the levels of the ESCRT-III CHMP2B after mitosis of the GSCs, and found that it increases progressively until abscission occurs. By contrast, no accumulation is observed in wild type germline cysts. In cysts lacking Usp8, CHMP2B levels accumulate with time (Figure 4A). In addition, we also noted a burst of Usp8 levels at cyst ring canals during each mitosis (Figure 4A). We therefore speculated that the long cell cycle of GSCs allows accumulation of ESCRT-III, while the short cell cycle in the cyst, combined with mitotic peaks of Usp8, prevents ESCRT-III accumulation and subsequent abscission (Figure 4A). To test experimentally this model, we used mutant flies with prolonged interphases specifically in germline cysts. In this background, cysts were initially devoid of ESCRT-III as in wild type cells (Figure 4B,C). However, when observed 10 h later, most cysts exhibited CHMP2B enrichment at their ring canals. After 10 more hours, the levels of CHMP2B increased and cysts were breaking apart. This result showed that time is a critical parameter for ESCRT-III localization, allowing their accumulation at ring canals and subsequent abscission.
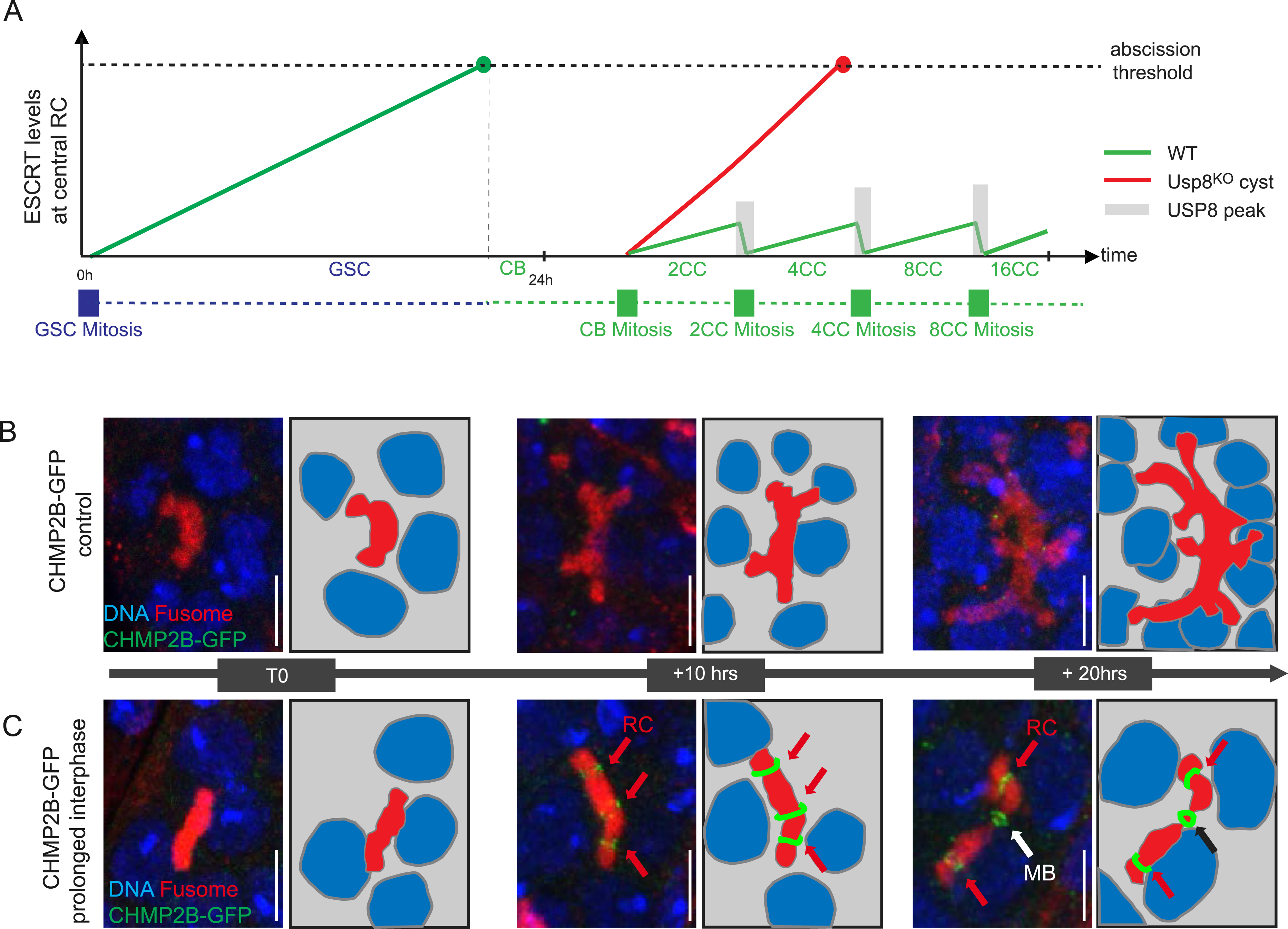
(A) Schematic model of ESCRT levels over time in the germarium. The GSC has a 24 h cell cycle. Abscission between the newly formed GSC and its daughter CB occurs late in the cell cycle, before the next mitosis, and so that the GSC/CB pair never experiences a Usp8 peak. During this long period, the GSC/CB RC accumulates ESCRT, reaching a high level allowing abscission. The CB becomes a 16-cell cyst in 24 h, therefore each cycle lasts only 6 h, leaving much less time to accumulate ESCRT at RC at each cycle. In addition, Usp8 levels at the RC in the cyst peak at each mitosis (grey bars) and remove (or block the dynamic recruitement of) ESCRTs at the RCs. We propose that short cell cycle duration combined with Usp8 peaks in mitosis are responsible for the low levels of ESCRT at cysts RC, and absence of abscission. When Usp8 is absent (red), ESCRT levels increase at each cyst cycle, allowing abscission. (B,C) Confocal images and schemes of fusomes (red) in germaria of females expressing CHMP2B-GFP in a WT context (B) or when cysts exhibit a prolonged interphase, from T0, to 10 and 20 h (C). In (C), 4-cell cysts are observed at T0 (left). CHMP2B-GFP is then observed at the RCs (red arrow) of a 4-cell cyst (+10 h, middle), and the central RC of a 4-cell cyst breaks into a MB (white arrow) at T0 +20 h (right). Scale bar: 5 μm. These results were originally described in [12]. Masquer
(A) Schematic model of ESCRT levels over time in the germarium. The GSC has a 24 h cell cycle. Abscission between the newly formed GSC and its daughter CB occurs late in the cell cycle, before the next mitosis, and so ... Lire la suite
Altogether, our results showed that the switch between complete and incomplete cytokinesis depends on the regulation of ESCRT-III ubiquitination levels by Usp8, coordinated with cell-specific cell cycle durations. These results were published in [12].
4. Perspectives
This work revealed that ubiquitination of ESCRT-III proteins promotes the completion of abscission in Drosophila GSCs, as it allows their accumulation at the abscission site. Interestingly, ubiquitination of ESCRT is also required for complete abscission in archeal cells, suggesting that this post-translational modification has a conserved role during evolution [47]. However, this ubiquitination leads to ESCRT degradation in archaea, whereas ESCRT are stabilized by ubiquitination in Drosophila germline cells. This raises the question of whether ESCRT stabilization by ubiquitination appeared with multicellular organisms. Choanoflagellates are the closest relatives to animals and have the ability to switch from unicellular form to multicellular colonies by incomplete divisions. Exploring the functions of Usp8 and ESCRT ubiquitination in choanoflagellates could thus reveal exciting insights into the origin of multicellularity.
Declaration of interests
The authors do not work for, advise, own shares in, or receive funds from any organization that could benefit from this article, and have declared no affiliations other than their research organizations.





 CC-BY 4.0
CC-BY 4.0