1 Introduction
In 1993, Sessoli et al. showed the existence of slow relaxation of the magnetisation at low temperature for the cage complex [Mn12O12(O2CCH3)16(H2O)4] [1]. This observation that a discrete cluster could show hysteresis of molecular origin has given birth to a new type of molecules called ‘single molecule magnets’ (SMMs). Since then, other manganese complexes [2] but also iron [3], vanadium [4] and more recently a dodecanuclear cyclic nickel complex [5] have shown this remarkable property. The interest in this type of compounds comes from their ability to store magnetic information and also in the fact that they can exhibit quantum tunnelling of the magnetisation [6]. Therefore, there is currently a great deal of interest in the synthesis of high-nuclearity clusters and one of the major challenges is to understand the correlations between the structures of the complexes and their magnetic properties.
In order to deal with this problem, our group is studying cobalt and nickel cages formed after ‘blend’ reactions of the metal salts with 2-pyridonate and carboxylates ligands [7, 8]. Recently, a family of these complexes has been reported with nuclearities from ten to twelve, the metal core of all these molecules being related to a trigonal prismatic geometry [7]. Benzoate was included in two of the clusters reported, an undecanuclear nickel cage with 2-chloro-6-pyridonate (chp) and a decanuclear cobalt compound with 2-methyl-6-pyridonate (mhp). We wanted to see if a modification of the solubility and/or the steric hindrance of the aromatic ring of the benzoate could influence the formation of new structures. Therefore we have used two substituted carboxylates, 2-methylbenzoate (o-MeBz) and 4-tert-butylbenzoate (p-tBuBz). Here we report the synthesis and the crystal structure of two decanuclear cobalt cages incorporating these ligands.
2 Experimental
All reagents, metal salts and ligands were used as obtained from Aldrich. Sodium salts of pyridone ligands were obtained by deprotonation of the corresponding hydroxypyridine in methanol using sodium methoxide followed by evaporation to dryness. Analytical data were obtained using an EA1108 elemental analyzer from Carlo Erba Instruments.
2.1 Synthesis of [Co10(OH)6(chp)6(o-MeBz)7-(n-C3H7OH)5][o-MeBz] 1
Hydrated cobalt nitrate (1.00 g, 3.4 mmol), Na(o-MeBz) (0.54 g, 3.4 mmol) and Na(chp) (0.52 g, 3.4 mmol) were added to MeOH (80 ml), and the solution stirred for 24 h before being filtered and evaporated to dryness under reduced pressure. The purple powder obtained was dried under vacuum for several hours and then extracted with n-propanol (20 ml) to give a pink solution which was filtered, and from which pink crystals of 1 grew from slow evaporation of the solvent in a 35% yield after four days. Elemental analysis calculated for C109H120O33N6Cl6Co10: C, 46.03; H, 4.25; N, 2.95; found: C, 45.74; H, 4.05; N, 3.10.
2.2 Synthesis of [Co10(OH)6(mhp)6(p-tBuBz)8-(Hmhp)2(CH3CN)2] 2
Hydrated cobalt nitrate (1.00 g, 3.4 mmol), Na(p-tBuBz) (1.37 g, 6.8 mmol) and Na(mhp) (0.89 g, 6.8 mmol) were added to MeOH (80 ml), and the solution stirred for 24 h before being filtered and evaporated to dryness under reduced pressure. The powder obtained was dried in vacuum for several hours and then extracted with acetonitrile (15 ml) to give a pink solution which was filtered, and from which pink crystals of 2 grew from diethyl ether diffusion in a 25% yield after 7 days. Elemental analysis calculated for C140H166O30N10Co10: C, 54.98; H, 5.47; N, 4.58; found: C, 54.77; H, 5.35; N, 4.32.
2.3 Structural studies
Crystal data, data collection and refinement parameters for compounds 1 and 2 are given in Table 1. Selected bond lengths and angles for these two complexes are given in Tables 2–5.
Experimental data for the X-ray diffraction studies of compounds 1 and 2.
| Compound | 1 | 2 |
| Formula | C109H120Cl6Co10N6O33 | C140H166Co10N10O30·C2H3N·3 H2O |
| M | 2844.11 | 3153.23 |
| Crystal system | monoclinic | orthorhombic |
| Space group | P21/n | Ccca |
| a (Å) | 16.202(10) | 32.75(2) |
| b (Å) | 28.91(3) | 34.68(2) |
| c (Å) | 27.973(10) | 29.352(19) |
| β (°) | 90.46(4) | |
| U (Å3) | 13103(16) | 33342(38) |
| T (K) | 293(2) | 150(2) |
| Z | 4 | 8 |
| ρcalcd (g cm–3) | 1.442 | 1.256 |
| Crystal shape and colour | pink plate | pink plate |
| Crystal size (mm) | 0.30 × 0.15 × 0.08 | 0.20 × 0.15 × 0.05 |
| M (mm–1) | 1.425 | 1.035 |
| F(000) | 5808 | 13104 |
| Theta range (°) | 1.89 to 25.04 | 1.71 to 25.02 |
| Limiting indices | 0 ≤ h ≤ 19, 0 k ≤ 34, –33 ≤ l ≤ 33 | –38 ≤ h ≤ 30, –41 ≤ k ≤ 40, –34 ≤ l ≤ 25 |
| Reflections collected / unique | 21197/21197 | 83608/14711 |
| Data / restraints / parameters | 21197/3197/1423 | 14711/467/783 |
| R1, wR2 | 0.0643, 0.1887 | 0.0841, 0.2358 |
| Goodness of fit | 0.942 | 0.871 |
Cobalt bond length ranges (Å) with e.s.d.s between parentheses for 1.
| Bonds involving central Co atom (Co1) | |
| Co–OH | 2.090(4)–2.125(4) |
| Bonds involving metal atoms capping square faces of trigonal prism (Co2, Co5 and Co8) | |
| Co–OH | 2.048(4)–2.096(4) |
| Co–O (bridging O2CR) | 2.012(5)–2.040(5) |
| Co–O (chp) | 2.183(4)–2.263(4) |
| Bonds involving metal atoms at vertices of trigonal prism (Co3, Co4, Co6, Co7, Co9 and Co10) | |
| Co–OH | 1.999(4)–2.034(4) |
| Co–O (bridging O2CR) | 2.000(5)–2.017(5) |
| Co–O (terminal O2CR) | 2.072(6) |
| Co–O (chp) | 2.172(5)–2.316(4) |
| Co–O (n-PrOH) | 2.111(5)–2.182(5) |
| Co–N (chp) | 2.111(6)–2.166(5) |
Cobalt bond angle ranges (°) with e.s.d.s. between parentheses for 1.
| Angles involving central Co atom (Co1) | |
| OH–Co–OH cis | 81.44(16)–106.86(16) |
| OH–Co–OH trans | 161.54(16)–161.95(16) |
| Angles involving metal atoms capping square faces of trigonal prism (Co2, Co5 and Co8) | |
| OH–Co–OH | 83.06(16)–83.27(17) |
| OH–Co–O (bridging O2CR) cis | 91.76(17)–94.48(18) |
| OH–Co–O (bridging O2CR) trans | 168.72(18)–171.86(17) |
| OH–Co–O (chp) | 75.78(16)–94.02(16) |
| O (bridging O2CR)–Co–O (chp) | 92.73(17)–98.24(19) |
| O (bridging O2CR)–Co–O (bridging O2CR) | 90.9(2)–92.02(19) |
| O (chp)–Co–O (chp) | 162.78(15)–163.84(14) |
| Angles involving metal atoms at vertices of trigonal prism (Co3, Co4, Co6, Co7, Co9 and Co10) | |
| OH–Co–O (bridging O2CR) | 94.45(18)–96.91(18) |
| OH–Co–O (chp) chelating metal site | 98.80(15)–102.66(16) |
| OH–Co–O (chp) bridging metal site | 76.11(16)–78.90(16) |
| OH–Co–O (n-PrOH) | 87.86(19)–92.5(2) |
| OH–Co–O (terminal O2CR) | 96.6(2) |
| OH–Co–N (chp) | 158.2(2)–163.40(19) |
| O (bridging O2CR)–Co–O (chp) chelating | 161.39(18)–163.71(17) |
| O (bridging O2CR)–Co–O (chp) bridging | 95.5(2)–98.5(2) |
| O (bridging O2CR)–Co–O (n-PrOH) | 94.1(2)–95.3(2) |
| O (bridging O2CR)–Co–O (terminal O2CR) | 93.4(2) |
| O (bridging O2CR)–Co–N (chp) | 101.2(2)–104.8(2) |
| O (chp) chelating–Co–O (chp) bridging | 78.37(16)–81.68(17) |
| O (chp) chelating–Co–O (n-PrOH) | 91.2(2)–92.60(19) |
| O (chp) chelating–Co–O (terminal O2CR) | 94.9(2) |
| O (chp) chelating–Co–N (chp) | 59.29(19)–60.99(17) |
| O (chp) bridging–Co–O (n-PrOH) | 162.27(19)–167.4(2) |
| O (chp) bridging–Co–O (terminal O2CR) | 169.0(2) |
| O (chp) bridging–Co–N (chp) | 94.87(19)–99.63(18) |
| O (n-PrOH)–Co–N (chp) | 90.2(2)–91.8(2) |
| O (terminal O2CR)–Co–N (chp) | 88.8(2) |
Cobalt bond length ranges (Å) with e.s.d.s between parentheses for 2.
| Bonds involving central Co atom (Co1) | |
| Co–OH | 2.089(7)–2.167(6) |
| Bonds involving metal atoms at vertices (Co2 to Co6) | |
| Co–OH | 1.987(6)–2.077(7) |
| Co–O (bridging O2CR) | 2.008(8)–2.125(7) |
| Co–O (chelating O2CR) | 2.128(7)–2.245(7) |
| Co–O (μ3-mhp) | 2.131(7)–2.272(7) |
| Co–O (μ2-mhp) | 2.031(7)–2.167(7) |
| Co–O (Hmhp) | 2.110(7) |
| Co–N (μ3-mhp) | 2.090(9)–2.135(9) |
| Co–N (μ2-mhp) | 2.166(9) |
| Co–N (MeCN) | 2.108(10) |
Cobalt bond angle ranges (°) with e.s.d.s between parentheses for 2.
| Angles involving central Co atom (Co1) | |
| OH–Co–OH cis | 80.1(4)–105.0(3) |
| OH–Co–OH trans | 160.1(2)–168.7(4) |
| Angles involving metal atoms at vertices (Co2 to Co6) | |
| OH–Co–OH | 81.7(4)–83.8(3) |
| OH–Co–O (bridging O2CR) cis | 93.8(3)–102.2(3) |
| OH–Co–O (bridging O2CR) trans | 168.9(3)–174.8(3) |
| OH–Co–O (chelating O2CR) | 89.3(3)–94.6(3) |
| OH–Co–O (μ3-mhp) | 76.7(3)–106.5(3) |
| OH–Co–O (μ2-mhp) | 84.4(3)–98.7(3) |
| OH–Co–O (Hmhp) | 83.5(3)–91.4(3) |
| OH–Co–N (μ3-mhp) | 160.1(3)–167.4(3) |
| OH–Co–N (μ2-mhp) | 158.7(3) |
| OH–Co–N (MeCN) | 88.3(3) |
| O (bridging O2CR)–Co–O (bridging O2CR) | 80.8(3)–92.4(4) |
| O (bridging O2CR)–Co–O (chelating O2CR) | 97.4(3)–155.5(3) |
| O (bridging O2CR)–Co–O (μ3-mhp) | 90.3(3)–165.5(3) |
| O (bridging O2CR)–Co–O (μ2-mhp) | 94.4(3)–96.6(3) |
| O (bridging O2CR)–Co–O (Hmhp) | 91.3(3)–94.1(3) |
| O (bridging O2CR)–Co–N (μ3-mhp) | 93.6(4)–105.5(4) |
| O (bridging O2CR)–Co–N (μ2-mhp) | 88.6(3) |
| O (bridging O2CR)–Co–N (MeCN) | 95.3(4) |
| O (chelating O2CR)–Co–O (chelating O2CR) | 59.9(3) |
| O (chelating O2CR)–Co–O (μ2-mhp) | 105.3(3)–160.8(3) |
| O (chelating O2CR)–Co–N (μ2-mhp) | 88.1(3)–103.4(3) |
| O (μ3-mhp)–Co–O (μ3-mhp) | 78.0(3)–169.1(4) |
| O (μ3-mhp)–Co–O (μ2-mhp) | 90.1(3)–158.4(3) |
| O (μ3-mhp)–Co–O (Hmhp) | 169.3(3) |
| O (μ3-mhp)–Co–N (μ3-mhp) | 97.7(3)–104.9(3) |
| O (μ3-mhp)–Co–N (MeCN) | 98.2(4)–162.5(4) |
| O (μ2-mhp)–Co–N (μ3-mhp) | 93.3(3) |
| O (μ2-mhp)–Co–N (μ2-mhp) | 61.8(3) |
| N (μ3-mhp)–Co–N (MeCN) | 87.9(4) |
3 Results
3.1 Crystal structure of 1
The structure of the cationic part of [Co10(OH)6-(chp)6(o-MeBz)7(n-C3H7OH)5][o-MeBz] 1 is shown in Fig. 1. The compound crystallises in the monoclinic space group P21/n. The ten cobalt atoms of 1 form a centred-tricapped-trigonal prism bridged by hydroxide, carboxylate and pyridonate ligands. The central cobalt atom Co1 is bound to six μ3-hydroxide ligands, which are bridging to the nine remaining metals to form the tricapped-trigonal prism. The six metal atoms at the vertices of the trigonal prism (Co3, Co4, Co6, Co7, Co9 and Co10) are sharing one μ3-OH with the central cobalt and the metal atoms capping the rectangular faces of the prism (Co2, Co5 and Co8) are sharing two μ3-OH ligands with this same cobalt atom.
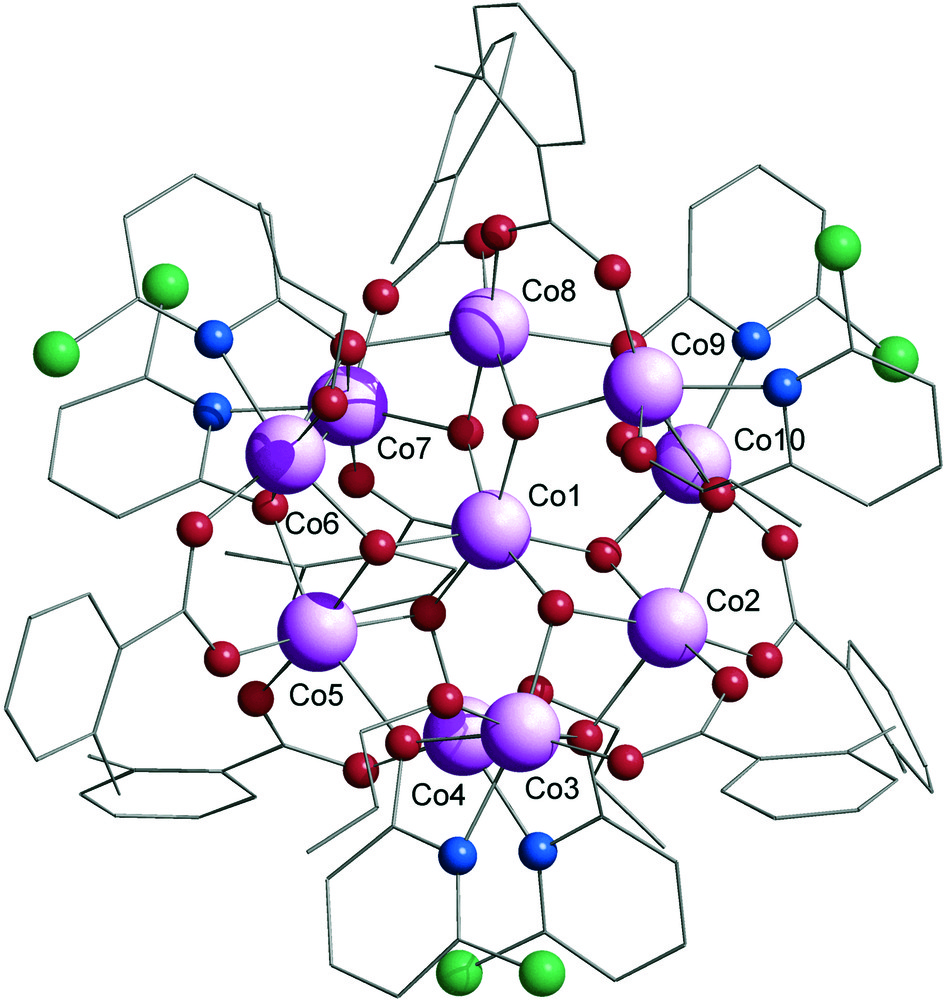
Structure of the cationic part of 1 in the crystal. Colour scheme: Co, pink; O, red; N, blue, Cl, green; C, grey.
Six chp ligands and six 2-methylbenzoate ligands bridge the exterior of the prism. Each chp ligand binds through its N-donor atom to one of the cobalt atoms at the vertices of the prism, and μ3-bridges through the O-atom. Following the Harris notation, the chp are adopting the 3.31 bridging mode. The Harris notation [9] describes the binding mode as [X·Y1Y2Y3...Yn], where X is the overall number of metals bound by the whole ligand, and each value of Y refers to the number of metal atoms attached to the different donor atoms. Therefore for both acetate and chp, there will be two values for Y. The ordering of Y is listed by the Cahn-Ingold-Prelog priority rules, hence O before N. The O-atom of chp is attached to: the metal vertex to which the N-atom is bound, the other vertex forming a side of the trigonal prism and a cobalt site capping a square face of the prism. The six O-atoms from the pyridonate ligand are then occupying the six triangular faces formed by the nine cobalt atoms: they are at the centre of the faces formed by a side of the prism and one of the caps.
Six of the seven 2-methylbenzoate ligands (o-MeBz) are bridging in a 2.11 fashion (Harris notation [9]) between cap and vertex sites, each cap being attached to two carboxylate ligands. To complete the structure, the last o-MeBz is in apical position to one of the metal vertex (Co7), at hydrogen bond distance of two n-propanol, whereas five n-propanol molecules from the crystallisation are attached to the five remaining cobalt atoms of the trigonal prism. The positive charge of the cluster is countered by the presence of an o-MeBz anion in the lattice.
The structure of 1 is based once again on the centred-tricapped-trigonal prismatic metal core [M10(μ3-OH)6(η2, μ3-xhp)6(η2, μ2-O2CR)6]2+ (several complexes have been previously obtained with M = Co or Ni, xhp = 2-chloro or 2-methylpyridonate and O2CR = chloroacetate, dimethylacetate, diphenylacetate, trimethylacetate or benzoate) [7]. Obviously, the substituted benzoate used does not induce the formation of a new cage structure, probably because it is not bulky enough. This is the first time that such a compound is obtained with cobalt when 2-chloropyridonate is used as the N-donor ligand. This novelty may be due to the improved solubility of the cage, provoked by the substituted benzoate. Using n-propanol as crystallisation solvent is not very common, which may also explain the isolation of 1.
3.2 Crystal structure of 2
The structure of [Co10(OH)6(mhp)6(p-tBuBz)8(Hmhp)2(CH3CN)2] 2 is shown in Fig. 2. The cluster crystallises in the orthorhombic space group Ccca. A two-fold symmetry axis passes through Co1 and Co5. The central cobalt atom Co1 is surrounded by six μ3-hydroxide ligands, which are bridging to the nine remaining metals. The 4-tertbutylbenzoate ligands are adopting two coordination modes: six of them are bridging between two cobalt atoms (2.11 mode) and each of the remaining two is chelating one metal (1.11 mode). The eight pyridonate ligands are adopting three coordination modes: four of them are bridging between three cobalt centres (in a 3.31 fashion according to Harris notation [9]), two other mhp are only bridging two metal sites (2.21 bridging mode) and each of the remaining two are linked through the oxygen to only one cobalt, which shows that they are in their protonated form: Hmhp. To complete this structure, two acetonitrile from the crystallisation solvent are also present, linked to two cobalt atoms through their nitrogen.
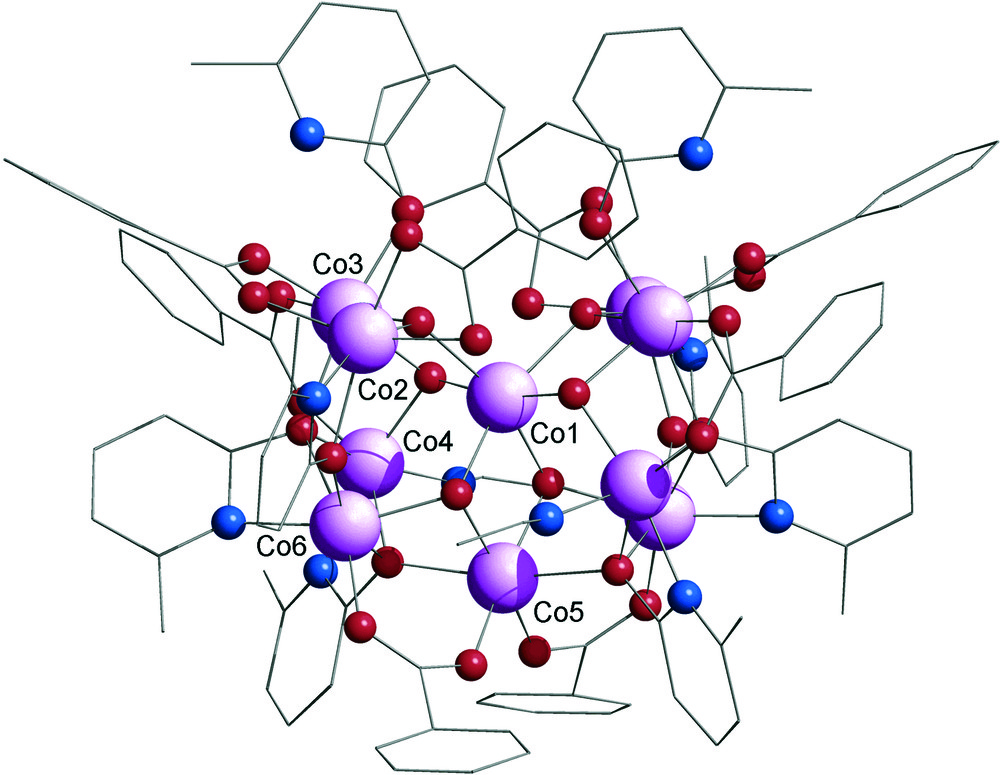
Structure of 2 in the crystal. Colour scheme as in Fig. 1.
The structure of 2 is not directly related to a trigonal prismatic metal core: the 4-tert-butylbenzoate ligand has induced the formation of a different cage structure. This type of core has been previously seen in three compounds when pivalate (piv) was used as the carboxylate in blend reactions [7]. The geometry of these three compounds of formula [M10(OH)4(xhp)10(piv)6(S)2] (where M = Ni or Co, xhp = chp or mhp and S = MeOH or EtOH) was related to a centred tetraicosahedron where five vertices are missing [10]. This structure has also been described as a centred pentacapped trigonal prism missing one edge of the prism [7]. This second description seems a little artificial, but it does recognize the similarity between structures such as 1 and 2.
While the core of 2 is similar to these previous examples its general formula differs from the three previous examples: two more hydroxides and two more carboxylates are present but there are only six pyridonate ligands instead of ten, and two protonated pyridonates are coordinated to 2. The three crystallographically distinct μ3-OH are respectively bridging between Co1, Co2 and Co3; Co1, Co3 and Co4; and Co1, Co5 and Co6. As for 1, the central cobalt is sharing two hydroxo bridges with Co5, Co3 and the symmetry equivalent of Co3. Three of the four crystallographically independent carboxylates act as 2.11-bridges between Co2 and Co3, Co3 and Co4, and Co5 and Co6, whereas the remaining p-tBuBz chelates Co2. Concerning the three independent mhp ligands: the first one is linked to Co4 through its nitrogen and bridges between Co4, Co5 and Co6 through its oxygen; the second one is nitrogen bounded to Co6 and the oxygen is linked to Co3, Co4 and Co6 and finally the nitrogen of the last one is linked to Co2 and its oxygen is coordinated to Co2 and Co6. The 2-methyl-6-hydroxypiridine (Hmhp) is oxygen bounded to Co3 and the acetonitrile from the crystallisation solvent is linked to Co4.
4 Conclusion
The use of the benzoate-based carboxylates o-MeBz and p-tBuBz in blend reactions with cobalt salts and pyridonates ligands has allowed us to isolate two new cobalt cages 1 and 2. In each case, the structure obtained has already been seen, but some differences have been observed due to the change in the size of the ligand and/or its solubility. We had previously assumed that it was the steric demands imposed by pivalate that led to the formation of the distorted core, as found in 2, in preference to the more regular trigonal prismatic core, as found in 1. However here the steric requirements imposed by the benzoate ligand substituted at the ortho position must be greater than the requirements of the para-substituted ligand but the structures found are reversed, i.e. the ‘more distorted’ core is found with the less sterically demanding ligand. This argues the structural control is subtler.
It is very easy to neglect reaction conditions when considering structure, and consider only the components found in the final crystal. However, here it may be we are seeing the influence of the relative solubilities imposed by differing carboxylates. The distorted tetraicosahedral core is only found when a t-butyl-group is found in the carboxylate – either directly attached (as in pivalate) or attached to a benzoate ligand. It seems possible that these highly solubilising groups are keeping the cages in solution longer, and hence leading to new topologies. We intend to hypothesise this theory by looking at other ligands featuring a tertiary butyl group. This hypothesis also suggests a new direction for study: use of ligands substituted to induce new solubilities, e.g. polyfluorinated carboxylates.
The use of more sterically demanding ligands, e.g., triphenylacetate lead to greater changes in the structures observed [11, 12]. At present, we can only take note of the differences and similarities between structures in order to understand the conditions that favour certain topologies. Then, hopefully, by varying parameters in the synthesis and comparing the results obtained, we will be able to control the properties of the cages formed.
5 Supplementary material
Full details of the crystal structure investigations have been deposited. CCDC-190087 and CCDC-190088 contain the supplementary crystallographic data for the structures reported in this paper, including atom coordinates, thermal parameters and remaining bond lengths and angles. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html, or on request from the Director of the Cambridge Crystallographic Data Centre, 12 Union Road, GB-Cambridge CB2 1EZ (UK) on quoting the full journal citation.
Acknowledgements
We thank the European Union for funding a TMR network on ‘Molecules as Nanomagnets’ contract No. HPRN-CT-1999-00012.


