1 Introduction
Mini-emulsion polymerisation is a process gaining more and more interest, due to the fact that, in this process, the nucleation step is greatly simplified versus the more conventional emulsion polymerisation process where the control of this nucleation step represents a major difficulty. Both processes need surfactants in order to stabilize the latex particles and control their size.
In the emulsion polymerisation, it is important to anchor the surfactant onto the surface of the particles in order to avoid its migration when the latex is used, either after flocculation, or for film formation in coatings. In the first case, one is faced with water pollution problems, and in the second case, with bad properties of the film upon exposure to moisture (lack of adhesion, water rebound, lack of dimensional stability). The best way to do that is to use polymerisable surfactants (surfmers), i.e., surfactants containing a polymerisable group. Then, it is important that the surfmer is polymerised at a high conversion in order to provide a good stabilization of the polymer particles. It has been suggested that mini-emulsion polymerisation might be an excellent mean of fully exploiting the potential of such polymerisable surfactants [1]. In this process, there is less surfactant in the serum, and the critical micellar concentration (CMC) is never reached, so that most of the surfactant is expected to be located at the surface of the droplets of monomer, which, upon polymerisation become polymer particles. The particle number is similar to the initial number of droplets and there is practically no change in the particle size, so that there is no growth step. Then the conversion at which the surfmer is copolymerised with the monomer is not important, provided the surfmer is not partitioned between the monomer phase and the particle surface. However, the suggestion to use surfmers in mini-emulsion polymerisation has never been applied experimentally except in the case of a study of mini-emulsion copolymerisation of vinylacetate and vinylethylhexanoate using an allylsulfosuccinate [2]. This study was used to demonstrate the interest of vinylethylhexanoate in mini-emulsion, and also the gain in nucleation efficiency obtained by the addition of polymer in the formulation. There are two other reports, by the same group [3], which deal with polymerisable co-stabilizers in mini-emulsion polymerisation. Our laboratory recently published a first study dealing with the use of the vinylbenzylsulfosuccinate [4] (VBSS), which has been also described in a recent paper [5].
The present paper is devoted to the study of two other polymerisable surfactants (surfmers) of the same series. One of these surfmers, sodium succinate of hydroxyethyl methacrylate (MAES), is now available commercially, while the second one, sodium succinate of hydroxybutylacrylate (ABS), is rather easy to prepare [5]. However, contrary to the case of VBSS, the mini-emulsion polymerisation failed when these surfmers were used alone, and thus mixtures with moderate amounts of a more conventional surfactant, sodium dodecylsulfate (SDS) are needed. In addition, the use of very simple hemiester (or hemiamide) for styrene mini-emulsion polymerisations using stearylmethacrylate (SMA) as co-stabilizer is also described. In that last part, as compared with the work reported in [3], the SDS was simply replaced with the hemiester (hemiamide) surfmer.
2 Experimental
2.1 Materials
All the experiments were done using deionized water. The synthesis of the surfactant has been described elsewhere [5]. The MAES surfmer is now commercially available (Aldrich) and was used without further purification. The butylacrylate (ABS) surfmer was prepared according to [5] by condensation of hydroxybutylacrylate on succinic anhydride, in dixoane solution at 80 °C in the presence of 4-dimethylaminopyridine (DMAP) as catalyst and hydroquinone as radical polymerisation inhibitor.
The hemiester (HEC12) and the hemiamide (Scheme 1 ) were prepared from linear C12 alcohol and amine respectively reacted with maleic anhydride, according to [6]. All other chemicals (SDS, non-ionic surfactants, ammonium persulphate, hexadecane (HD) and stearylmethacrylate (SMA), all from Aldrich) were used as received. The monomers, styrene and methylmthacrylate (MMA), also from Aldrich, was distilled under vacuum, and stored in a refrigerator under nitrogen atmosphere before use.
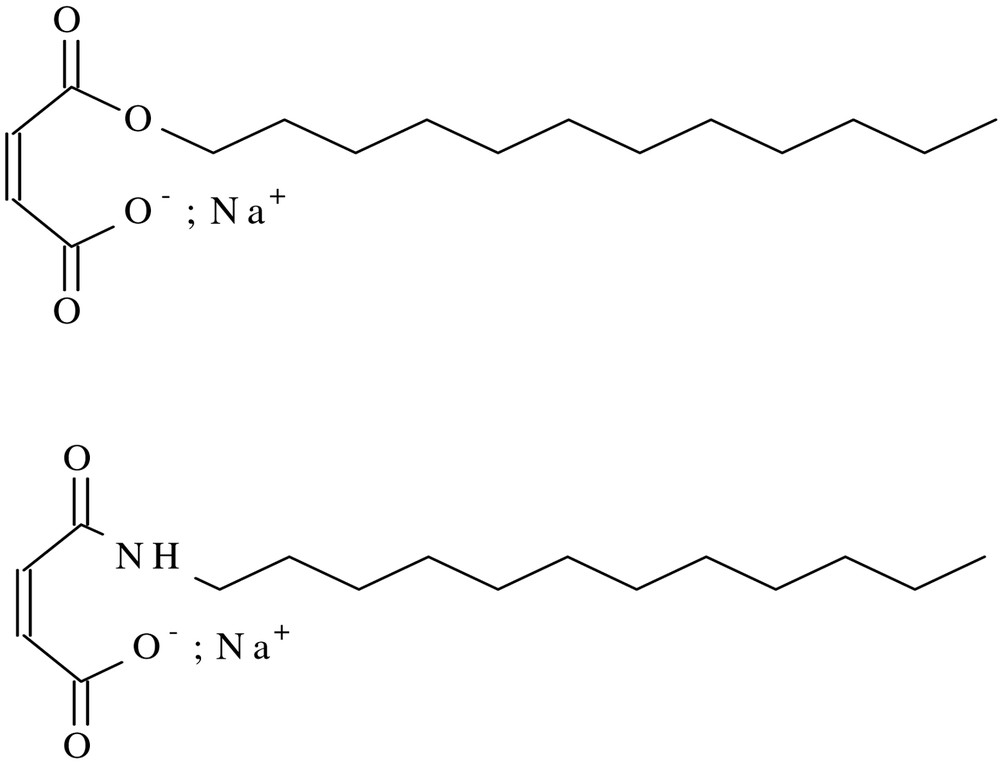
Structure of hemiester HEC12 and of the hemiamide HA.
The critical micellar concentration (CMC) was determined from surface tension measurements by the Wilhelmy plate method, using a KRUSS K 12 processor tensiometer.
2.2 Mini-emulsion preparation
The monomer and hexadecane or SMA, are first mixed and added to the water solution containing the surfactants (the carboxylic acid of the hemiesters being neutralized with stoichiometric amounts of soda). The mixture is then stirred magnetically for 30 min before being submitted for 2 min to ultrasound agitation (Ultrasonic processor Branson at 90% power) or 20 min with an Ultraturrax machine. The size of the droplets is measured (Malvern Autosizer), and the mini-emulsion is reputed stable when the size does not change within a few hours. The composition of the mini-emulsions of MMA using HD as hydrophobe, are reported in Table 1.
Mini-emulsion composition
| Surf./SDS% | Surf. G | Aqueous NaOH (g) | Phase SDS (g) | Water (g) | Organic MMA (g) | Phase HD (g) | Dd (nm) |
| MAES | 0.3180 | 0.055 | 0 | 50 | 12.15 | 1.02 | 597 |
| 100/0 | |||||||
| 0/100 | 0 | 0 | 0.151 | 50 | 12.4 | 1.00 | 105 |
| 86/14 | 0.225 | 0.34 | 0.0375 | 50 | 12.3 | 1.02 | 123 |
| 67/33 | 0.154 | 0.025 | 0.0743 | 50 | 12.2 | 1.01 | 307 |
| 40/60 | 0.075 | 0.013 | 0.1125 | 50 | 12.5 | 0.98 | 224 |
| 35/65 | 0.068 | 0.0107 | 0.125 | 50 | 12.05 | 0.99 | 133 |
| ABS | 0 | 0 | 1 | 100 | 20 | 2 | 58.5 |
| 0/100 | |||||||
| 100/0 | 1 | 0 | 100 | 20 | 2 | 1250 | |
| 50/50 | 0.5 | 0.5 | 100 | 20 | 2 | 110 | |
| 75/25 | 0.75 | 0.25 | 100 | 20 | 2 | 85 |
2.3 Polymerisation of the mini-emulsions
The polymerisations are carried out under nitrogen atmosphere in a glass reactor (250 ml) under moderate stirring (250 rpm) at 65 °C. Most often, ammonium peroxydisulphate (APS: 0.2 g, dissolved in 5 ml of water) is used as initiator. Polymerisation kinetics are followed, by sampling at given times, and determining the conversions by gravimetry. Particle sizes were determined by quasi-elastic light scattering (Malvern Autosizer). Conductimetric measurements were also carried out at the end of the polymerisations. Size exclusion chromatography (SEC) was carried out, after redissolution of the dried materials in tetrahydrofurane (THF). In addition to the estimation of the polymer molecular weight, such SEC analysis allows the detection of the amount of unconverted surfmer.
2.4 Latex characterization
The latexes are washed four times by passing over ion-exchange resins. Conductimetric measurement of the strong SO4– and weak COO– acid groups with NaOH followed by back titration with HCl, according to [7], allows us to calculate the amount of surfactant that is grafted onto the surface of the particles or so strongly adsorbed that it is not eliminated by the washing procedure, and then, from the surface area of the spherical particles, it is possible to calculate the charge density onto the particle surface. The latex can be further dialysed through a cellulosic membrane, in order to separate oligomers of moderate molecular weight, which are not actually grafted, but only strongly adsorbed. A part of the surfmer is consumed to produce such co-oligomers with MMA. Taking into account the unconverted surfmer, separated in the SEC measurement, it is then possible to determine the balance of the surfmer.
2.5 Latex stability
The stability of the latexes was tested by determining the resistance to addition of electrolytes; 1ml of latex is mixed with 10 ml of a solution of electrolyte (MgSO4, 0.1 M); the particle size was measured before and 48 h after the test. The same was done to determine the freeze-thaw stability: the latex was kept at –20 °C for one night and then warmed again at room temperature and the particle size was measured. Finally, in some cases a flocculation test in the presence of an equivalent volume of acetone was carried out. If the mixture remains stable, a new particle diameter measurement was done.
3 Results and discussion
3.1 CMC of the surfmers
According to Uzulina et al. [5], the CMC of the surfmers alones is rather high, respectively 12.5 and 3.9 g l–1 for MAES and ABS. However surface tension measurements show a continuous decrease of γ rather than a true CMC, chiefly in the case of MAES, and it is somewhat difficult to estimate the CMC. The value of γ remains higher than 50 mN m–1. These surfmers did not display good surface activity and can be considered as well as functional monomers. However, in case of mixtures with SDS, a CMC close to that of SDS is observed with surface tensions at and above the CMC of about 30 mN m–1, i.e. a value again close to the one observed with SDS alone. The mixed micelles of 75% ABS and 25% SDS seem to behave like the micelles of SDS alone.
The CMC of HEC12 is 0.9 g l–1, lower than that of the SDS (2.3 g l–1).
3.2 Formation of mini-emulsions
A series of stable mini-emulsions have been prepared easily, according to the formulations reported in Table 1 with mixtures of SDS and reactive acrylic surfmers. When the MAES or ABS surfmers are used alone the size of the droplets is big (close to 1 micron) and the mini-emulsion is not stable for an enough a long time.
It has been possible to prepare stable mini-emulsion of MMA using mixtures of the surfmers with non-ionic surfactants, either Triton (an ethoxylated nonylphenol) in the case of MAES, or Disponil (an ethoxylated long linear alcohol chain). The corresponding data will be reported together with the results of the mini-emulsion polymerisations. Of course, it may be argued that the measurement of the droplet sizes using dynamic light scattering can be considered as questionable, because such measurements involve an important dilution of the mini-emulsion of the monomer, which has a rather high water solubility. However, there is no safe method, according to a recent review [8], but the light scattering method is, by far, the simplest one.
3.3 Polymerisation of MMA mini-emulsions
The results of polymerisation of the MMA–HD mini-emulsions are reported in Table 2 for the experiments carried out with mixtures of surfactants containing MAES, and in Table 3 for those containing APS.
Polymerisation of MMA Mini-emulsions stabilized using MAES mixtures with either SDS or Triton
| MAES(%) | 34 | 34 | 58 | 58 | 75 | 75 | 86 | 86 | 37 (triton) | 37 (triton) |
| Min | X% | Dp (nm) | X% | Dp (nm) | X% | Dp (nm) | X% | Dp (nm) | X% | Dp (nm) |
| 0 | 0 | 112 | 0 | 111 | 0 | 128 | 0 | 137 | 0 | 214 |
| 10 | 13.7 | 102 | 40 | 87 | 64 | 109 | 53 | 112 | 7.4 | 215 |
| 20 | 31.5 | 95 | 92 | 105 | 94 | 111 | 96 | 132 | 15.7 | 234 |
| 30 | 93.6 | 107 | 94 | 105 | 97 | 105 | 99 | 137 | 20 | 248 |
| 45 | 97.7 | 104 | 97 | 104 | 98 | 111 | 98 | 132 | 35 | 260 |
| 60 | 99 | 103 | 91 | 105 | 99 | 109 | 102 | 136 | 63 | 337 |
| 90 | 99 | 102 | 100 | 106 | 100 | 108 | 101 | 132 | 69 | 316 |
Polymerisation of MMA mini-emulsions stabilized using ABS mixtures with either SDS or Disponil
| ABS% | 75 | 50 | ||||
| SDS% | 25 | |||||
| DISPO.% | 50 | |||||
| Time min. | X% | Dp (nm) | Np (1016) | X% | Dp (nm) | Np (1015) |
| 0 | 97 | 7.5 | 0 | 195 | 9.3 | |
| 30 | 94 | 94 | 7.6 | — | — | — |
| 60 | 94 | 96 | 7.8 | 76 | 205 | 7.9 |
| 90 | 94 | 99 | 8.0 | 79 | 207 | 5.9 |
In the case of MAES, regardless the composition of the surfactant system, the polymerisation reactions are fast and complete conversions are reached within about 20–30 min. This is true except when the second surfactant is the non-ionic Triton; in this case, the size of the particles is larger and there is a trend towards limited flocculation, because the particle size tends to increase (from 215 to 320 nm). When the surfactant system is fully anionic, there are only slight changes in the particle size, which tends to decrease.
Even though fewer experiments were carried out with ABS, the same trends are valid: high polymerisation rates, complete conversion and almost no change in the particle size, when the surfactant system is fully anionic. Bigger particles, and trend towards a limited flocculation and lower polymerisation rate are observed when the surfactant system does include a non-ionic surfactant. In the later case a limited conversion that levels off is observed.
3.4 Polymerisations of styrene using hemiester (HEC12) or hemiamide (HA)-SMA systems
The results of a first series of experiments carried out for the mini-emulsion polymerisations of styrene, using the simple hemiester surfmer are reported in Table 4. The mini-emulsion droplets include also 0.1% of KPS. Upon polymerisation at 70 °C, some latexes become unstable and flocculate. In the other case, a latex with bimodal particle size distribution is formed. The origin of the second population may be caused by excess surfactant, as in runs 2,3,4, because the surfactant can be liberated into the serum and cause renucleation, if it is very dynamic with a low CMC (0.09 g l–1).
Styrene mini-emulsion polymerisations initiated with potassium persulphate at 70 °C.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| H2O | 81.49% | 80.47% | 78.10% | 78.40% | 80.94% | 81.15% | 80.91% |
| NaOH | 0.04% | 0.11% | 0.10% | 0.10% | 0.11% | 0.05% | 0.06% |
| HEC 12 | 0.54% | 1.39% | 1.30% | 1.20% | 1.18% | 0.66% | 0.83% |
| SA | 0.54% | 0.86% | 1.70% | 1.60% | 0.57% | 0.62% | 0.79% |
| Styrène | 17.38% | 17.17% | 18.80% | 18.70% | 17.19% | 17.52% | 17.42% |
| D.drops (nm) | 125. | 170. | 216. | 225. | 120. | 159. | 152 |
| PDI | 0.30 | 0.60 | 0.07 | 0.08 | 0.25 | 0.10 | 0.20 |
| D.particles (nm) | 131. | 83. | 150. | 165. | 80. | 142. | 127. |
| PDI | 0.15 | 0.24 | 0.40 | 0.45 | 0.30 | 0.30 | 0.22 |
| Covering rate% Sc = 53 Å2 per molecule | 66 | 234 | 253 | 244 | 140 | 100 | 123 |
| Covering rate% Sc = 30 Å2 per molecule | 37 | 132 | 143 | 139 | 79 | 57 | 69 |
| Remarks | floculation | bipopulation | bipopulation | bipopulation | floculation | not stable | not stable bipopulation |
The distribution of the particle sizes has been studied. In run 2, there was probably too much surfactant; the distribution is bimodal with a population of large particles corresponding probably to the initial droplets, while the excess of surfactant gave rise to a population of smaller particles. For run 5, there is an indication of a trimodal distribution with a possible formation of flocculated particles from not enough stabilized particles, while some liberation (desorption) process might be responsible for the appearance of the smallest particles in a fast polymerisation process. The distribution is monomodal for run 6, but the final latex is not stable.
In a second series of experiments, a small amount of PMMA was added in the recipe. It is known that such addition may improve the stability of the mini-emulsions [9]. In addition, the hemiamide HA12 is compared to the hemiester HEC12.
Regarding the formation of mini-emulsions, the presence of PMMA allows to reduce the droplet size with a narrower polydispersity.
Comparison of the two surfmers shows that HEC12 leads to bigger particles, but with a narrower distribution. Upon polymerisation in the presence of PMMA, renucleation is observed after about 10% conversion, as reported in Table 5, dealing with the evolution of the particle size with the conversion for runs 8 and 9.
Styrene polymerisations using hemiester (run 9) or hemiamide (run 8) in the presence of PMMA as hydrophobe
| Time (min) | Dn (nm) | Run 8 (Ip) | % conv. | Dn (nm) | Run 9 (Ip) | % conv. |
| 0 | 466 | 0.5 | 0 | 215 | 0.16 | 0 |
| 5 | 500 | 0.47 | 9 | 212 | 0.16 | 3.3 |
| 15 | 530 | 0.65 | 11 | 204 | 0.15 | 7.1 |
| 30 | 294 | 0.82 | 16.2 | 170 | 0.3 | 33.1 |
| 60 | 320 | 0.88 | 44.6 | 138 | 0.3 | 62.8 |
| 120 | 108 | 0.25 | 81.3 | |||
| 180 | 122 | 0.46 | 59 |
In both cases, the renucleation phenomenon corresponds to an acceleration of the polymerisation, while, in the case of the hemiamide, there is a trend for limited flocculation during the whole procedure. It seems that the polymerisation rate is proportional to the surface area offered for the capture of the radicals produced only in the water phase. Then the trend for limited flocculation might explain the limited yield in the case of the hemiamide. The latex produced in run 9 is stable for weeks. When after dilution, it is washed by passing it through ion exchange resins, it flocculates after five successive treatments when the conductivity begins to decrease. The same treatment applied in case of run 8 (hemiamide) leads to faster flocculation. The reproducibility of run 9 was tested and was found to be approximately good (initial droplet size 285 instead of 215-final particle size after 120 min, 99 nm instead of 108, conversion 88% instead of 81). Only slightly more renucleation took place. High molecular weight (Mw = 960 000) and high polydispersity (4.3) have been observed, probably more typical for an emulsion polymerisation than for a mini-emulsion process, supposed to be closer to a bulk polymerisation. Because of the quite continuous renucleation, it was not possible to distinguish between the effects of both mechanisms. The composition of the polymer, as studied by proton NMR after separation from the serum, indicate that both the styrene and the acrylate are about fully polymerised, while the conversion of HEC12 is rather limited
3.5 Behaviour of the surfmers
Upon polymerisation, the surfmers are expected to copolymerise with the main monomer MMA, so they become grafted at the particle surface. However, as component of the surfactant system, their molecules are expected to be partitioned between the particle surface and the water phase. In addition, these surfmers, and specially MAES, are highly water-soluble and are expected to be present in the water phase in rather high concentration. Then, after reaction with the radicals formed upon thermal decomposition of APS they should produce, together with the MMA molecules copolymers with rather high molecular weights; a part of the latter species could be adsorbed onto the polymer particle surface.
Conductivity measurements were carried out all along the polymerisation. No changes in the serum conductivity have been observed. After that, we attempted to separate the serum from the particles by means of high-speed centrifugation. These trials were unsuccessful, because even at more than 20 000 rpm, no clear serum separated. The supernatant liquid was always opalescent, with tiny particles remaining in suspension. Conductimetric titration of the acid groups present onto the polymer particle surface were carried out, after purification of the latexes by passing through a mixture of anion and cation exchange resins, and titration with soda and further back-titration with HCl. It was possible to titrate both the strong acid sulphate groups, coming from the initiator, and the weak acid carboxylic groups coming from the grafted surfmers. In one case, the purification procedure was completed by dialysis through a cellulosic membrane. The results are reported in Table 6.
Conductimetric titration of acidic groups onto the surface of latex particles
| Latex | μequiv SO4– (g) | Dc (μequiv cm–2) | μequiv COO–/g | Dc (μequiv cm–2) | % MAES onto surface |
| MAES 86% | 4.3 | 1.73 | 52.4 | 12.6 | 64 |
| SDS 14% | |||||
| Dialysed | 4.0 | 0.86 | 38.0 | 9.6 | 47 |
| MAES 75% | 3.3 | 1.1 | 36 | 7 | 54 |
| SDS 25% | |||||
| MAES 58% | 3 | 1.0 | 25.4 | 5.1 | 56 |
| SDS 42% | |||||
| MAES 34% | 5.3 | 1.0 | 22.7 | 4.3 | 100 |
| SDS 66% |
The amount of strong acid groups coming from the initiator APS is rather limited, and is hardly sufficient to ensure the stability of the latex. Indeed the limiting value for the PMMA latex stability that can be obtained from emulsion polymerisation of MMA without emulsifier has been observed to be about 1.7 μC cm–2. However, the charge density due to the weak acid groups, due to the grafting of the MAES, is very much larger and enough to ensure the latex stability. Depending on the concentration of MAES in the surfactant system, a more or less important part of it is found on the surface; that part is quite large when the concentration is low, and decreases when the concentration increases (from 100% when the proportion of SDS is 66%, to 64% if the concentration of SDS is limited to 14%). In the latter case, that part decreases from 64 to 47% upon dialysis, showing that 17% of the corresponding MAES species belong to compounds strongly adsorbed but not actually grafted; we believe that these species are most probably co-oligomers of MMA and MAES, which are able to cross the cellulosic membrane. In order to obtain more information about the surfmer behaviour, a sample of the final latex was dried, redissolved in tetrahydrofurane and analysed by SEC. A complex peak of high molecular weight polymer can be observed in the SEC diagram, followed by a small peak (383 in Fig. 1 ) at an elution time of 26 min, corresponding well to the elution time of the pure MAES; a semi-quantitative estimate corresponds to about 35% of the initial amount of the MAES added. Thus, in this case, 47% remains grafted, 17% forms oligomers strongly adsorbed, and 35% remains unconverted in the serum. The complex peak of the SEC diagram might correspond partly to these co-oligomers and partly to the high polymer inside the polymer particles. Unfortunately, we did not succeed in further analysing this peak, due to the fact that attempts to analyse separately the serum were not successful.
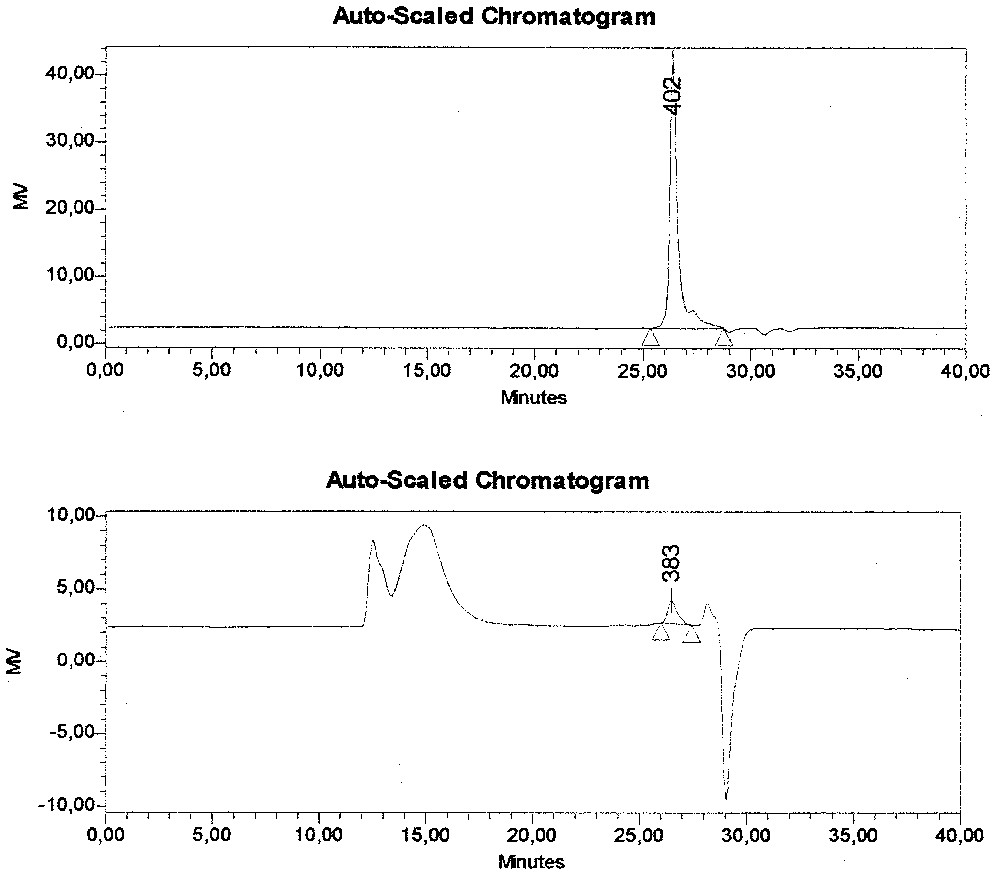
SEC chromatograms of MAES (up) and latex from MMA mini-emulsion polymerisation, using 86% MAES and 14% SDS as surfactant system (below).
Conductimetric titrations have been also carried out in the case of the experiment using 75% ABS and 25% SDS. It was concluded that, in this case, about 14% of the ABS remained grafted at the surface of the latex particles.
In the case of styrene polymerisations, using HEC12 as surfactant, addition of a non-ionic surfactant (Rewopol H25) was done, and five successive treatments with ion exchange resins were carried out. Upon conductometric titration, only 25% of the initial charge was left on the surface. It was observed that, without protection by a non-ionic surfactant, the latex flocculates after five resin treatments.
3.6 Latex stability
As shown by the results reported in Table 7, when submitted to the stability tests, such as the acetone test or the freeze-thaw tests the latexes prepared using MAES are most often stable. In only one case, with the lower amount of MAES, the acetone test causes flocculation; in all the other cases, the diameter after the test is quite similar to the initial one. About similar results have been obtained for the freeze-thawing test. In that case, no case of catastrophic flocculation has been observed, but increasing the amount of MAES causes a decrease in the difference between the initial diameter of the particles and the one observed after the test; limited flocculation does occur, but to the lesser extent for the higher concentration of polymerisable surfactant.
Stability tests
| MAES% | SDS% | Dp (nm) | Acetone | Freeze |
| 34 | 66 | 102 | Floc | 196 |
| 58 | 42 | 108 | 107 | 168 |
| 75 | 25 | 141 | 107 | 162 |
| 86 | 14 | 136 | 136 | 151 |
| 37 | 63 triton | 322 | 311 | nd |
In the case of ABS, even with a limited amount of grafted surfmer, the freeze-thaw test is positive, the diameter increasing from 167 to 221 nm. Concerning the stability versus electrolyte addition, the same latex show a good stability up to 0.5 N concentration of KCl.
In the case of styrene polymerisation using HEC 12 as surfactant and PMMA as hydrophobe (run 9 of Table 5), a washing treatment was done by diluting one volume of the latex in one volume of a mixture 1:1 of ethanol and water. The mixture is then centrifuged, and redispersed in the 1:1 mixture; after this treatment has been repeated, the particle size was measured again and found to be 96 nm instead of 99 initially. Titration of the carboxylic groups shows that 92% of the initial charge of HEC12 remains located on the surface.
4 Conclusion
Two acrylic surfmers, the condensate of hydroxyalkyl acrylic monomers onto succinic anhydride, thus easy to prepare or even commercially available, have been used successfully in the polymerisation of MMA mini-emulsions; these mini-emulsions however must be stabilized by mixtures of the surfmers with a limited amount of a more conventional anionic or non-ionic surfactant. High polymerisation rates, and high conversions of the MMA were observed with SDS as complementary surfactant. With non-ionic surfactants, slower rates and sometimes limited conversions were obtained. These surfmers are however highly water-soluble, and show rather poor surface activity. Thus they behave more as water-soluble monomers than surfactants. Then, a part of them remains in the water phase and are not polymerised. Another part is producing water-soluble copolymers with MMA, which are strongly adsorbed onto the particles, but not actually grafted. However, the major part remains grafted in proportion higher when the amount of SDS in the initial mixture of surfactant is the higher. Finally, the rather high grafting efficiency of these acrylic surfmers does confer to the latexes a good stability versus the usual tests, specially the freeze-thawing test, which is not so common with purely anionic surfactants.
Although the system styrene–stearylacrylate–C12 hemiester of maleic acid (sodium salt) seems to be able to form rather stable mini-emulsions upon polymerisation, it does not give the expected result (i.e. keep the size of the droplets for the polymer particles).
Instead of that, renucleation is most often observed, and sometimes flocculation takes place.
Moreover, only a limited part of the reactive surfactant is actually located on the surface. However, that surfactant seems to be rather strongly adsorbed onto the surface of the particles, and little is left unreacted in the serum.


