1 Introduction
In the synthesis of latexes, useful in the production of waterborne coatings (paints, adhesives, textile seizers, binders for paper coatings, etc.), the use of reactive surfactants, and especially of polymerizable surfactants (surfmers) allows a certain number of benefits, in terms of latex stability and behaviour of the films when exposed to water or moisture (less water rebound, dimensional stability, maintenance of adhesive properties) [1–3]. Among these surfmers, maleic acid derivatives have been shown to be rather close to the optimum behaviour [4]. For that reason, our group has engaged a rather systematic study of the chemistry of maleic surfactant synthesis, together with their application in emulsion polymerization, for the preparation of polystyrene latexes and film-forming copolymers [5–10]. This work has been reviewed recently [11].
The present paper is a continuation of this study, using maleic diamide surfactants derived from the chemistry of isoimides [12]. Recently, a family of maleic acid diesters with hydrophilic and hydrophobic groups has been successfully used as surfmers for preparation of in-such-a-way stabilized latexes [5–10]. Replacement of maleic diesters by diamides seems to be a promising goal because of the increased stability of amide bonds against hydrolysis and increased hydrophilicity of amides by comparison with the corresponding esters.
Conversion of maleic hemiamides (HA) into the corresponding diamides demands an activation of the carboxylic group before the amide bond formation. The problem is similar to that in peptide synthesis. Maleic isoimides (1, MII) might be regarded as an alternative activated form to well-known mixed anhydrides, activated esters, and other popular intermediates for amide synthesis from their precursors – HA. They might facilitate very much conversion of HA into the desired diamides (3).
MII (1) are easy to prepare by cyclization reactions of HA in the presence of water-consuming and activating reagents – dicyclohexyl carbodiimide (DCC), ethyl chloroformate – triethyl amine, etc. Methods for preparative syntheses of MII with variable substituents at nitrogen atom have become available recently [12]. Exploitation of MII in preparations of different maleic acid diamides would permit to avoid or minimize undesirable side reactions – addition to C=C double bond, isomerization to the corresponding fumaric acid derivatives, etc. Therefore conversion of MII into new maleic surfmers is discussed more closely in this paper. The paper describes the synthesis of a number of such diamides, with a variety of hydrophobic groups, as well with a variety of hydrophilic groups. It can be considered that this is a new and easier way to obtain reactive maleic surfactants, the behaviour of which is not far from the optimal, as shown by Schoonbrood and Asua [4]. Some of these compounds have been engaged in emulsion polymerization, after having characterized their colloidal properties (CMC).
2 Experimental
2.1 Surfactant synthesis
2.1.1 N-Dodecyl-N′-(2-hydroxyethyl)-maleic diamide (3c)
Method A. 2-Aminoethanol (0.24 g, 4 mmol) in DMFA (5 ml) was added drop by drop to the stirred and cooled (–5…0 °C) solution of N-dodecyl maleic isoimide (1a) (1.06 g, 4 mmol) in DMFA (10 ml). After stirring at –5 °C for two more hours, the stirred mixture was brought to room temperature and stirred for additional 2 h. DMFA was distilled off in vacuo, the residue was recrystallized from acetonitrile. White crystals (0.8 g, 62%) of 3c with m.p. 88–89 °C were obtained after drying in vacuo.
Method B. 2-Aminoethanol (1.83 g, 30 mmol) in CH2Cl2 (10 ml) was added drop by drop to the stirred and cooled (–5…0 °C) solution of N-dodecyl maleisoimide (1a) (7.96 g, 30 mmol) in CH2Cl2 (20 ml). After addition, the mixture was allowed to warm up to room temperature and stirred for additional 2 h. Precipitate was separated and recrystallized from acetonitrile. White crystals (8.2 g, 84%) of 3c with m.p. 88–89 °C were obtained after drying in vacuo.
Other maleic diamides (3a–l) were prepared in a similar way. Their chemical characterization is presented in Table 1, and their spectroscopic data in Tables 2 and 3. Sugar derivative (3f) is prepared from amino sugar salt in slightly different way, as described below.
Characterisation of the maleic diamides (3)
| Compound | Yield (%) | m.p. (°C) | Found (%) | Formula | Calc. (%) | ||||
| 3a | 52* | 67–69 | 73.85 | 10.00 | 7.44 | C23H36N2O2 | 74.15 | 9.74 | 7.52 |
| 3b | 31* | 62–64 | 67.13 | 10.66 | 8.07 | C19H36N2O3 | 67.02 | 10.66 | 8.23 |
| 3c | 62* 84** | 88–89 | 66.40 | 10.68 | 8.52 | C18H34N2O3 | 66.22 | 10.50 | 8.58 |
| 3d | 43* 71** | 46–48 | 64.52 | 10.63 | 7.52 | C20H38N2O4 | 64.83 | 10.34 | 7.56 |
| 3e | 80** | 40–42 | 67.87 | 11.24 | 11.71 | C20H39N3O2 | 67.95 | 11.12 | 11.89 |
| 3f | 20* | 110 a | 59.24 | 9.31 | 6.20 | C22H40N2O7 | 59.44 | 9.07 | 6.30 |
| 3g | 91** | 92–93.5 | 57.54 | 8.91 | 12.32 | C11H20N2O3 | 57.87 | 8.83 | 12.27 |
| 3h | 76*** | oil | 55.70 | 8.67 | 12.94 | C10H18N2O3 | 56.06 | 8.47 | 13.07 |
| 3i | 50*** | 103–105 | 55.89 | 8.70 | 13.03 | C10H18N2O3 | 56.06 | 8.47 | 13.07 |
| 3k | 62*** | 131–133 | 53.47 | 4.93 | 10.33 | C12H13ClN2O3 | 53.64 | 4.88 | 10.43 |
NMR spectroscopic characteristics of the maleic diamides (3)
| Compound | Solvent | 13C NMR, δ (ppm) | 1H NMR, δ (ppm) | |||||
| =O | = | CONH– | H2–O/H2–N | C=C | N–CH2 | NH–C2 | ||
| 3a | CDCl3 | 164.93 | 132.73 | 43.67 | – | 6.09* | 8.62* | 3.27 |
| 164.83 | 132,36 | 39.93 | 7.87* | 4.55(Bz) | ||||
| 3b | d6-DMSO | 164.53 | 132.06 | 38.61 | 70.20 | 6.13* | 9.46(t) | 3.11 |
| 164.26 | 131.37 | 38.49 | 9.33(t) | ~ 3.3 | ||||
| 3c | d6-DMSO | 164.82 | 131.78 | 41.69 | 59.54 | 6.14 | 9.23** | 3.10 |
| 164.32 | 131.72 | 38.72 | 6.08 | 3.19 | ||||
| 3d | d6-DMSO | 164.72 | 132.14 | 39.00 | 68.79 | 6.16* | 9.55(t) | 3.13 |
| 164.51 | 131.84 | 38.89 | 9.40(t) | 3.33 | ||||
| 3e | d6-DMSO | 164.61 | 131.81 | 38.59 | 57.60 | 6.13 | 9.28(t) | 3.10 |
| 164.26 | 131.50 | 36.59 | 6.10 | 9.20(t) | 3.24 | |||
| 3f | d6-DMSO + D2O | 164.98 | 132.09 | 54.64 | masked | 6.19 | ~ 9.15 | 3.09 |
| 164.64 | 131.95 | 6.11 | 3.2–3.8 | |||||
| 3g | d6-DMSO | 164.96 | 132.55 | 50.04 | 59.41 | 6.18 | 9.50(t) | 2.96 |
| 164.35 | 131.17 | 41.68 | 6.13 | 9.09(t) | 3.20 | |||
| 3h | CDCl3 | 166.07 | 132.44 | 47.20 | 61.60 | 6.14 | 8.85(t) | 3.87 |
| 164.24 | 132.39 | 42.70 | 6.09 | 8.25(d) | 3.46 | |||
| 3i | CDCl3 | 166.23 | 134.04 | 51.46 | 61.21 | 6.09 | 8.73(t) | –3.45 |
| 164.34 | 131.20 | 42.47 | 6.03 | 8.26(s) | ||||
| 3k | CD3OD | 167.94 | 133.35 | 138.35 | 61.33 | 6.32 | – | –3.39 |
| 165.14 | 133.14 | 43.15 | 6.28 |
IR spectroscopic characteristics of the maleic diamides (3)
| Compound | Solvent | IRS, ν (cm–1) | |||
| N–H | =C–H | amide I | amide II | ||
| 3a | nujol | 3310 | covered by nujol | 1635 | 1540 |
| 1620 | |||||
| 3b | nujol | 3280 | 3060 | 1670 | 1545 |
| 1620 | |||||
| 3c | nujol | 3280 | 3060 | 1680 | 1550 |
| 3250 | 1630 | ||||
| 3d | film | 3270 | 3060 | 1660 | 1550 |
| 1620 | |||||
| 3e | nujol | 3290 | 3070 | 1630 | 1565 |
| 3090 | 1550 | ||||
| 3g | CHCl3 | 3280 | 3060 | 1670 | 1565 |
| 1620 | |||||
| 3h | film | 3270 | 3060 | 1670 | 1550 |
| 1620 | |||||
| 3i | nujol | 3390 | 3060 | 1650 | 1565 |
| 3240 | 1620 | ||||
| 3k | nujol | 3270 | 3080 | 1640 | 1560 |
| 1615 |
2.1.2 N-Dodecyl-N′-(2-dezoxy-2-D-glucosyl)-maleic diamide (3f)
Method C. N-Dodecyl maleic isoimide (1a) (2.65 g, 10.0 mmol) was added to a fine suspension of D-glucosamine hydrochloride (2.16 g, 10.0 mmol) in dry dimethylformamide, cooled in ice bath. Triethyl amine (1.4 ml, 10 mmol) was slowly added to the reaction mixture. The mixture was allowed to warm up to room temperature, and stirred 24 h at room temperature, then 5 h at 80 °C. Dimethylformamide was distilled off in vacuo to leave a dark viscous residue, which was recrystallized three times from acetonitrile-tetrahydrofurane (with addition of charcoal) to yield white-off powder of 3f (0.89 g, 20%) (Tables 1–3).
2.1.3 N-Dodecyl-N′-[2-(N-allyl-N,N-dimethylammonio)ethyl]-maleic diamide chloride (5a)
Solution of maleic diamide 3e (2.96 g, 8.7 mmol) and allyl chloride (0.71 ml, 8.7 mmol) in acetonitrile (15 ml) was kept at room temperature for two days and then refluxed for 1 h. The hot solution was filtered and the solvent was distilled off in vacuo. The residue was triturated with dry ether to yield white-off powder of 5a (2.30 g, 62%). Compound 5b was obtained as sticky oil in a similar way (Tables 4–6).
Characterisation of alkylated dimethylaminoethyl maleic diamides (5, 7)
| Compound | Yield (%) | m.p. (°C) | Found (%) | Formula | Calc. (%) | ||||
| C | H | N | C | H | N | ||||
| 5a | 62* | 108–110 | 62.00 | 10.59 | 9.95 | C23H44N3O2Cl | 64.23 | 10.31 | 9.77 |
| 5b | 68* | oil | 61.10 | 7.20 | 12.06 | C18H26N3O2Cl | 61.44 | 7.45 | 11.94 |
| 5c | 61* | 176–177 | 65.93 | 7.28 | 10.56 | C22H28N3O2Cl | 65.74 | 7.02 | 10.45 |
| 5d | 82* | 230–232 | 61.41 | 9.17 | 7.87 | C27H47N3O5S | 61.68 | 9.01 | 7.99 |
| 5e | 70** | 90–91 | 59.15 | 6.73 | 9.13 | C22H29N3O5S | 59.04 | 6.53 | 9.39 |
| 7a | 81* | 220–222 | 58.45 | 9.41 | 8.62 | C23H45N3O5S | 58.07 | 9.54 | 8.83 |
| 82** | |||||||||
| 7b | 72** | 148–150 | 54.61 | 7.14 | 10.43 | C18H27N3O5S | 54.39 | 6.85 | 10.57 |
NMR spectroscopic characteristics of maleic diamides with quaternary ammonium groups (5, 7)
| Compound | Solvent | 13C NMR, δ (ppm) | 1H NMR, δ (ppm) | ||||
| =O | = | CONH– | H2–N+ | C=C | N+(C3)n | ||
| 5a | CD3OD | 168.84 | 133.18 | 40.58 | 63.03 | 6.18 (s) | 3.09 (s) |
| 166.62 | 131.76 | 34.39 | |||||
| 5b | CD3OD | 168.81 | 133.44 | 44.29 | 63.05 | 6.28 (s) | 3.10 (s) |
| 166.62 | 131.55 | 34.40 | |||||
| 5c | CD3OD | 168.96 | 133.67 | 44.17 | 63.67 | 6.29 (s) | 3.07 (s) |
| 166.55 | 131.29 | 34.52 | |||||
| 5d | CD3OD | 168.75 | 133.10 | 40.56 | 65.64 | 6.21 (s) | 3.18 (s) |
| 166.60 | 131.80 | 34.71 | |||||
| 5e | d6-DMSO | 165.67 | 132.48 | 42.18 | 63.60 | 6.23 (s) | 3.09 (s) |
| 164.22 | 129.99 | 33.05 | |||||
| 7a | CD3OD | 168.72 | 133.02 | 40.59 | 64.41 | 6.23 | 3.17 (s) |
| 166.65 | 131.94 | 34.39 | 63.18 | 6.20 | |||
| 7b | CD3OD | 168.81 | 133.40 | 44.17 | 64.41 | 6.28 (s) | 3.14 (s) |
| 166.68 | 131.58 | 34.40 | 63.16 |
IR spectroscopic characteristics of maleic diamides with quaternary ammonium groups (5, 7)
| Compound | Solvent | IRS, ν (cm–1) | |||
| N–H | =C–H | amide I | amide II | ||
| 5a | CHCl3 | 3220 | 3030 | 1650 | 1550 |
| 1620 | |||||
| 5b | CHCl3 | 3280 | 3030 | 1670 | 1560 |
| 1630 | |||||
| 5c | CHCl3 | 3220 | 3030 | 1660 | 1545 |
| 1630 | |||||
| 5d | nujol | 3240 | 3040 | 1670 | 1560 |
| 1620 | |||||
| 5e | CHCl3 | 3270 | 3030 | 1655 | 1545 |
| 1625 | |||||
| 7a | nujol | 3250 | 3040 | 1645 | 1555 |
2.1.4 N-Benzyl-N′-[2-(N-benzyl-N,N-dimethylammonio)ethyl]-maleic diamide chloride (5c)
Solution of maleic diamide 3l (6.88 g, 25 mmol) and benzyl chloride (3.0 ml, 25 mmol) in acetonitrile (20 ml) was kept at room temperature for two days and then refluxed for 1.5 h, and left at room temperature for one day. The solvent was distilled off in vacuo. The residue was triturated with dry ether to yield white-off powder of 5c (6.25 g, 61%), (Tables 4–6).
2.1.5 N-Dodecyl-N′-[2-(N,N,N-trimethylammonio)ethyl]-maleic diamide 4-methyl benzene sulphonate (5d)
Solution of maleic diamide 3e (3.1 g, 13.1 mmol) and methyl 4-methyl benzene sulphonate (2.4 g, 13.1 mmol) in acetonitrile (20 ml) was kept at room temperature for seven days. The solvent was distilled off in vacuo. The residue was carefully ground with dry ether to yield, after drying in vacuo, white-off powder of 5d (5.8 g, 82%) (Tables 4–6).
2.1.6 N-Benzyl-N′-[2-(N,N,N-trimethylammonio)ethyl]-maleic diamide 4-methyl benzene sulphonate (5e)
This compound was prepared in a similar way as 5d.
2.1.7 N-Dodecyl-N′-{2-[N,N-dimethyl-N-(3-sulfopropyl) ammonio]ethyl}-maleic diamide (7a)
Solution of diamide isoimide 3e (1.0 g, 2.8 mmol) and 1,3-propane sultone (0.3 g, 2.8 mmol) in acetonitrile (8 ml) was refluxed for 2 h, cooled and kept at –5 °C for 24 h. A precipitate of 7a was obtained (1.1 g, 81%). The compound 7b was obtained in a similar way (Tables 4–6).
The other reactive surfactant (dodecylmaleamide), was prepared from maleic anhydride and linear C12 primary amine, according to [9].
2.2 Other materials
Most of the compounds used in the polymerization were commercial products (Aldrich) used as received. The monomers, styrene and butyl acrylate were distilled under vacuum and stored, before use, under nitrogen atmosphere. Non-reactive surfactants were either sodium dodecyl sulphate (SDS) or cetyl trimethyl ammonium bromide (CTAB). The initiators were, either potassium persulphate (KPS) or the cationic 2,2′-azobis (2-amidinopropane)dihydrochloride (AIBA).
2.3 Colloidal properties of the surfactants
The critical micellar concentrations (CMC) were determined from surface tension measurements by the Wilhelmy plate method, using a KRUSS K 12 processor tensiometer.
2.4 Styrene batch polymerization
In a typical experiment, the polymerization was performed under nitrogen atmosphere at 70 °C in a glass reactor equipped with a stirrer rotating at 315 rpm. The reactor was fed with water (160 g) and surfactant (0.4 g), and left for degassing for 30 min at 70 °C. Then styrene (40 g) was added, and the polymerization was started upon introduction of 0.15 g of K2S2O8 in 5 g of water. Samples were taken at time intervals to determine the conversion by gravimetry and follow the changes in particle size. In most cases, the conversion of the monomer was completed after 5 h, and then the polymer was collected for further characterization.
2.5 Core-shell latexes preparation
A seed of polystyrene latex was prepared at 70 °C, under nitrogen atmosphere, in a 1-l reactor equipped with a stirrer rotating at 250 rpm. It was fed with 900 ml of deionized water containing 1.5 g of SDS and 1 g of NaHCO3, and 100 g of distilled styrene. The polymerization was started upon the addition of 0.75 g of KPS dissolved in 5 g of water. The conversion was completed in 20 h. The seed latex was then washed by passing two times through ion-exchange resins. The particle size of that monodisperse seed latex was 98 nm.
A similar seed with a particle diameter of 87 nm was prepared using the cationic surfactant CTAB and the cationic initiator AIBA.
About 0.5 g of surfmer dissolved in 75 g of deionized water at 70°C were introduced in the reactor of 250 ml. 50 g of the seed latex was added. After degassing under nitrogen, 2 g of a mixture of styrene and butyl acrylate was added and the system was left overnight at room temperature. After that swelling step, a solution of surfmer and initiator was added, and the temperature allowed to reach 70 °C, and the polymerization was started by simultaneous addition of the monomer mixture (2.4 ml min–1) and a solution of the remaining surfactant with 0.15 g of initiator. The addition was completed after 5 h, and then another portion of initiator was added and the polymerization was completed after 8 to 20 h. The amount of monomers added is calculated so that the targeted final size is 150 nm. Aliquot samples were taken from time to time in order to follow the process, and the conversion was determined by gravimetry.
At the end of the polymerization, the surface tension of the latex was measured, using the same equipment as that for the CMC analysis of the surfactants.
2.6 Polymer characterization
The amount of coagulum was determined upon filtration of the latex using a sintered glass filter, and after washing the reactor with tetrahydrofurane (THF); both the THF solution and the product collected on the filter were dried and the sum of the solids was taken as the amount of coagulum.
Particle-size measurements were carried out by dynamic light scattering with Autosizer LO-C (Malvern Instrument) giving the average size and the particle size distribution. In a few cases, TEM micrographs were also used.
Molecular weights were measured by Size Exclusion Chromatography (SEC), using a Waters instrument, Porapack gel filled columns, and polystyrene calibration standards.
2.7 Latex stability tests
The latexes were tested for their stability versus addition of electrolytes, addition of ethanol and versus freeze-thawing cycles.
To 1 g of latex, 1 g of 0.1 M NaCl solution was added. Flocculation was observed immediately if a higher concentration of salt was used.
The ethanol test consists in adding to a volume of latex an equal volume of ethanol, allowing desorbing the organic species not firmly adsorbed or anchored onto the surface of the particles. After this treatment, the latex is diluted and the particle size is measured, except if flocculation was observed.
For a freeze-thawing test, a small amount of latex (1–2 ml) was kept at –20 °C for one day, and after another day at room temperature the flocculation was observed, and, if not, the particle size was measured and compared with the initial particle size of the latex.
3 Results and discussion
3.1 Surfactants synthesis
Maleic isoimides (MII, 1) are highly reactive intermediates in syntheses of various maleic acid derivatives.
Reactions of MII (1) with nucleophilic reagents - amines or amino alcohols (2) would allow preparing a set of corresponding maleic diamides (MD, 3) with highly hydrophilic substituents at one of the amide groups. MII (1) with hydrophobic substituents at nitrogen atom were used (Scheme 1 ) in order to introduce hydrophobic part at the second amide group in the planned surfmers – MD (3). These MD (3) represent a new group of potential surfmers with variable hydrophobic/hydrophilic balance caused by different substituents at both nitrogen atoms. They are very much more resistant against hydrolysis by comparison with earlier described surfmers – maleic acid diesters in alkaline media –, which is essential for copolymerization reactions.
Reactions of MII with amines were carried out in polar solvents, best of all DMFA or dichloromethane. Low reaction temperatures (~0 –5 °C) are necessary to avoid undesirable side reactions (addition to the C=C double bond, isomerization into fumaric acid derivatives, etc.). Usually amines are added to maleisoimides (1) solutions, but amines also can be generated in situ from their salts, as it is demonstrated in case of preparation of an amino sugar derivative (3f) from its salt. The obtained MD (3) have particular structure – both their hydrophilic NH groups are placed next to polymerizable C=C double bond. It means that the C=C double bond might be placed on phase surface or even in water phase during the exploitation in polymerization of these surfmers. Albeit yields of MD (3) are variable (30–90 %), the method seems to be among the best ones for large-scale preparation of maleic surfmers, mainly due to higher stability of starting materials (MII) by comparison with other activated forms of HA. Conditions of synthesis and characteristics of the obtained MD are collected in Table 1.
Structures of synthesized MD (3) were conformed by their NMR and IR spectra. Signals of protons at the C=C double bond (δ ~ 6.0…6.2), amide NH protons (δ ~ 8.3…9.5), and those of CH2–N groups (δ ~ 3.1…3.4 ppm) differ substantially from the starting materials (isoimides, 1). Coupling constants of protons at the C=C double bond (J = 8…12 Hz) confirm the fact that isomerization to the corresponding fumaric derivatives has not taken place during their synthesis, provided that reactions (Scheme 2 ) were performed on sufficient cooling. In 13C NMR spectra of MD (3), the most characteristic resonance signals are those of carbon atoms in C=C double bond (131–134 ppm) and C=O groups (164–168 ppm). IR spectra of diamides (3) have the most characteristic intensive absorption bands at 1615–1670 (amide I), 1540–1560 (amide II), 3250–3310 (νN–H) and 3060–3080 (ν=C–H) cm–1. Spectral characteristics of the MD are summarized in Tables 2 and 3.

Investigated MD (3) contain short-chain alkyl groups (R1 in 3) with increased hydrophilicity, due to the presence of extra hydroxyl-, ether- or amino-groups in R1. Selection of the right group allows obtaining predictable balance between hydrophobic and hydrophilic part of the surfmer molecule, which, in turn, influences total surface activity of the surfmer.
Further increase of the hydrophilicity of some MD (3) was achieved by N-alkylation of MD, provided that they contain external amino group (3e, 3l). Alkylation with alkyl halides or sulphonates (4) leads to the corresponding ammonium salts (5) – cationic surfmers –, while alkylation with 1,3-propane sultone (6) allows preparing aminosulfonic acids – zwitterionic surfmers (7). All alkylation reactions proceed in polar aprotic solvents at 20…80 °C, and yields of the mentioned ammonium salts (5, 7) are high enough for preparation of these surfactants in preparative scale (Table 4).
Once more, prepared ionic surfactants (5, 7) were characterized by NMR and IR spectra. Apart from resonance signals of parent molecule protons (at C=C double bond, of the group NH–CH2–, etc.), 1H NMR spectra of the ammonium salts (5) contain characteristic triplet of CH2–CH2–NR3 group at ~3.4 ppm, but zwitterionic derivatives (7) – signals at ~ 3.2 and ~ 2.9 ppm of protons next to N and S correspondingly in ≡N+–(CH2)3–SO3– group.13C NMR spectra of 5 and 7 apart from resonance signals of C atoms in double bonds have other characteristic signals of C atoms next to quaternary ammonium groups (~ 65 ppm). IR spectra of the compounds (5) and (7) have strong characteristic absorption bands of amide groups at 1620–1670 (amide I), 1545–1560 (amide II) cm–1, as well as those of N–H bonds at 3220–3270 and =C–H bonds at 3030–3040 cm–1. Unfortunately, it is not easy to find something particular for the introduced hydrophilic groups of 5 and 7 in these spectra. The most characteristic spectral data of these ionic surfmers are collected in Tables 5 and 6.
All examined maleic diamides (3, 5, 7) are efficient surfactants indeed, and they also contain activated (by two amide groups) polymerizable C=C double bonds.
3.2 Colloidal properties
The CMC of the three classes of surfmers are reported in Table 7, together with the values of the surface tension at the CMC. The values of these CMC are rather small, in between 1 and 10 × 10–5 mol l–1. Such values are in the range of common non-ionic surfactants, but they indicate a rather poor hydrophilicity of the molecules, possibly because the hydrophilic moieties are rather short. However, the surface tension values at the CMC, in between 25 and 50 mN m–1 indicate rather good surface activity.
Colloidal properties of maleic diamide surfmers
| Surfmer | CMC (μmol l–1) | CMC (mg l–1) | T (°C) | γ at CMC (mN m–1) |
| 3d | 82 | 27.8 | 22 | 28.7 |
| 3c | 44 | 14.3 | 21 | 48.2 |
| 3g | 93 | 41 | 21 | 36.1 |
| 7a | 150 | 70.5 | 21 | 30.5 |
| 5a | 86 | 37 | 22 | 32.7 |
| 5d | 180 | 99 | 22 | 44.7 |
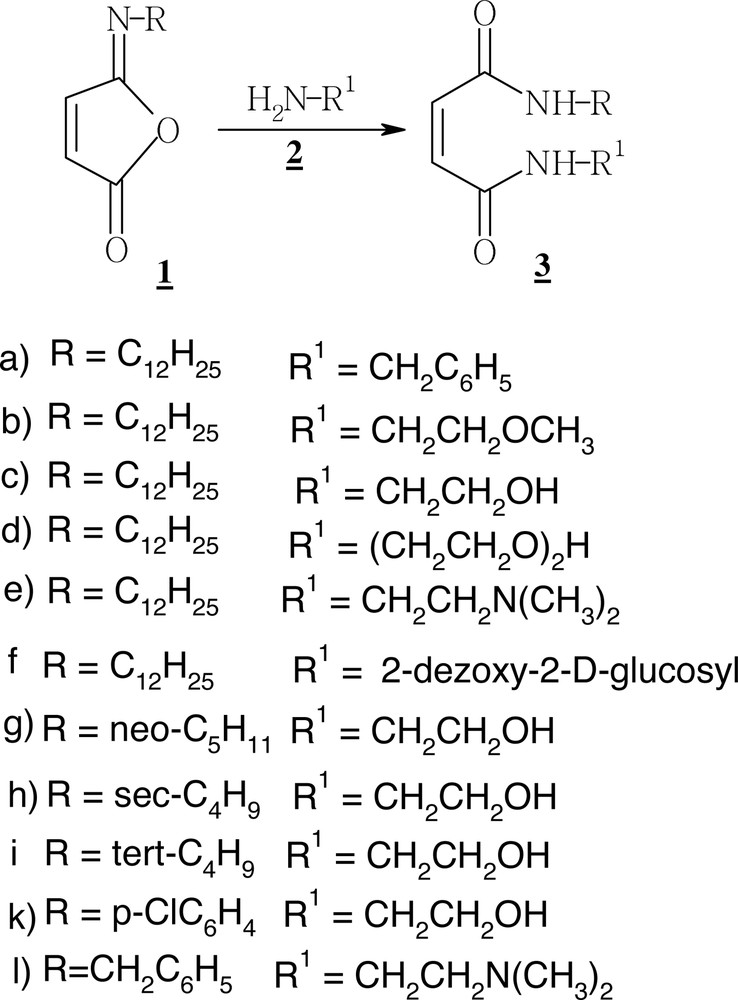
An example of determination of the CMC is shown in Fig. 1 .
Surface tension versus log c, for water solutions of the non-ionic diamide surfmer 3d at 22.5 °C.
3.3 Batch emulsion polymerization of styrene
When applied in batch emulsion polymerization of styrene, the non-ionic surfmers used alone without co-surfactant are unable to cause high conversion of the monomer in small polymer particles. In fact, they are not able to provide a successful nucleation step in the polymerization process. So it is needed to associate with them an anionic surfactant such as SDS. As soon as the surfactant system does include SDS, very good results can be obtained: high conversion in a rather limited time, with a narrow population of small particles and very little amount of coagulum. Most of the surfactants remain attached or at least strongly adsorbed onto the particle surface, as shown by the measurement of the surface tension of the final latex, close to the value of pure water. The replacement of SDS with the reactive anionic surfactant HA12 gives the same result. High molecular weight was obtained with a broad distribution, as usual in emulsion polymerization. These data are reported in Table 8, while a few conversion curves are shown in Fig. 2 .
Batch emulsion polymerization of styrene (surfactant: 0.3 g, co-surfactant: 0.1 g, styrene/water: 20%, T = 70 °C, KPS: 0.15 g)
| Surfactant | Co-surfactant | D (N m) | PDI | Conv. (%) | Coag. (%) | γ (mN m–1) | time (min) | Mn (× 103) | Mw/Mn |
| 3d | SDS | 215 | 0.05 | 90.5 | 2.4 | 72.3 | 300 | 275 | 3.7 |
| 3d | SDS | 140 | 0.06 | 100 | 1.8 | 74 | 210 | ||
| 3d | HA12 | 141 | 0.08 | 100 | 1.4 | 72.8 | 180 | 352 | 3.2 |
| 3c | SDS | 179 | 0.06 | 100 | 2.2 | 73.6 | 240 | 723 | 5.1 |
| 3c | SDS | 128 | 0.07 | 94.5 | 0.9 | 74.7 | 210 | 1318 | 4.2 |
| 3g | SDS | 82 | 0.08 | 100 | 1.8 | 73.3 | 180 | 259 | 4.7 |
| 5d | – | 95 | 0.04 | 99.7 | 1.6 | 72.6 | 170 | 8.8 | 17.4 |
| 5e | – | 178 | 0.37 | 7.6 | 300 | 5.6 | 24.6 | ||
| 5a | – | 88 | 0.06 | 99.7 | 1.3 | 73.6 | 180 | ||
| 7a | – | 199 | 0.05 | 99.9 | 1.8 | 73.8 | 270 | 200 | 3.8 |
| 7b | – | >400 | 1.0 | 25 | 390 |
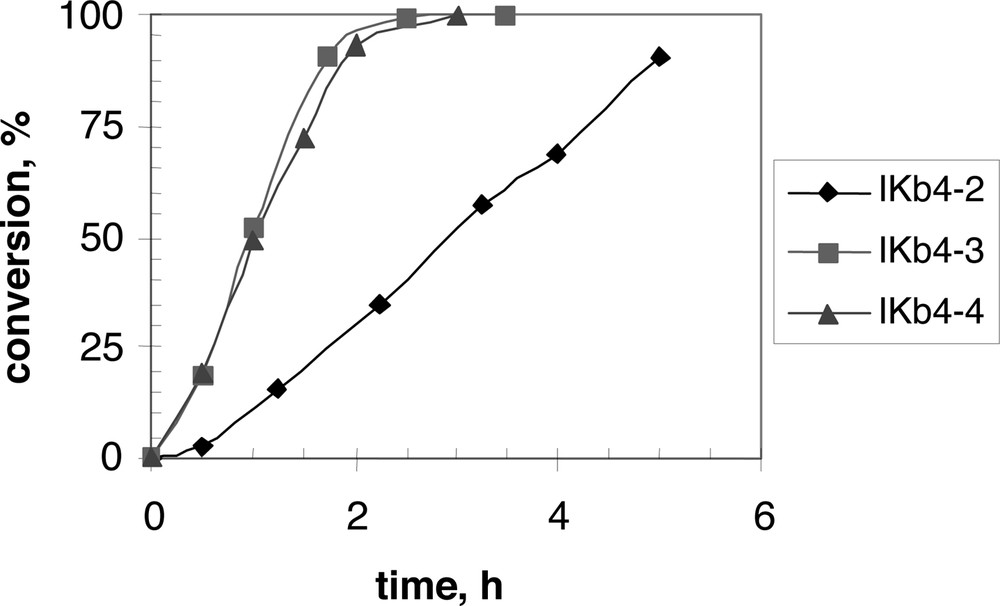
Conversion versus time for batch emulsion polymerization of styrene, using the non-ionic diamide surfmer 3d alone (IKb4-2) or together with SDS (IKb4-3) or HA12 (IKb4-4) as anionic co-surfactants at 70 °C.
Further data about the cationic surfactants (5) and the zwitterionic surfmers (7) are also reported in Table 8. In these cases, the ionic nature of the surfactants allows to obtain an efficient nucleation, so that there is no need for a co-surfactant. However, with the compounds 5e and 7b, poor polymerization yields are obtained. The larger particle sizes are an indication that the nucleation is less efficient, but it seems that the main cause of these poor results has to be found in the benzylic structure of the hydrophobic part. It is well known that the benzylic groups are very sensitive, in radical polymerization to transfer reactions, leading to delocalized radicals (in the benzene ring) with low reactivity [13]. Otherwise, when the hydrophobic part is an alkyl group, as in the case of the previous non-ionic surfmers, the results of the polymerization are very good, chiefly in the case of the cationic surfmers, leading to even smaller particle sizes. However the molecular weights are very small in the case of the cationic surfmers, most probably due to the counterions. The zwitterionic compound 7a leads to somewhat slower polymerization with larger particle size, but again with high molecular weight and usual polydispersity.
3.4 Core-shell semi-batch seeded polymerization
A smaller number of surfmers with only alkyl hydrophobic moiety were engaged in a seeded semi-batch copolymerization. The corresponding results are reported in Table 9.
Seeded semi-batch emulsion copolymerization of a 1:1 mixture of styrene and butyl acrylate
| Surfmer | D (N m) | PDI | Conv. (%) | Coag. (%) | Surface tension (mN m–1) | Mn (× 103) | Mw/Mn |
| 3d | 152 | 0.04 | 97.8 | 0.8 | 64.9 | 198 | 4.1 |
| 3c | 149 | 0.04 | 94.7 | 2.1 | 64.7 | 140 | 4.8 |
| 5d | 175 | 0.06 | 95.9 | 1.3 | 60.1 | 16.7 | 23.8 |
| 5a | 170 | 0.05 | 97.9 | 1.6 | 60.1 | 9.7 | 43 |
| 7a | 149 | 0.05 | 99.3 | 0 | 65.1 | 161 | 4.6 |
In all cases, monodisperse particles with convenient sizes have been obtained. The conversion is almost complete, with very small amounts of flock. At the end of the polymerization, it remains a significant amount of surface active compounds. This feature may be surprising, because the conversions reach high values in all cases. Most probably, because the reactivity of styrene is somewhat higher than that of butyl acrylate, the very surface of the particles is composed chiefly of butyl acrylate units, which have adsorption properties quite different from polystyrene. This remark involves idea that the contribution of the grafting of the surfmer units should be limited as compared to the adsorption of these surfmers. Such behaviour was already observed in the case of surfmers derived from maleic hemiesters [7]. Again, the molecular weights measured in the case of the cationic surfmers are much lower and polydisperse than of other polymers.
Fig. 3 shows an example of conversion curves (both instantaneous and global) in the semi-batch copolymerization process.
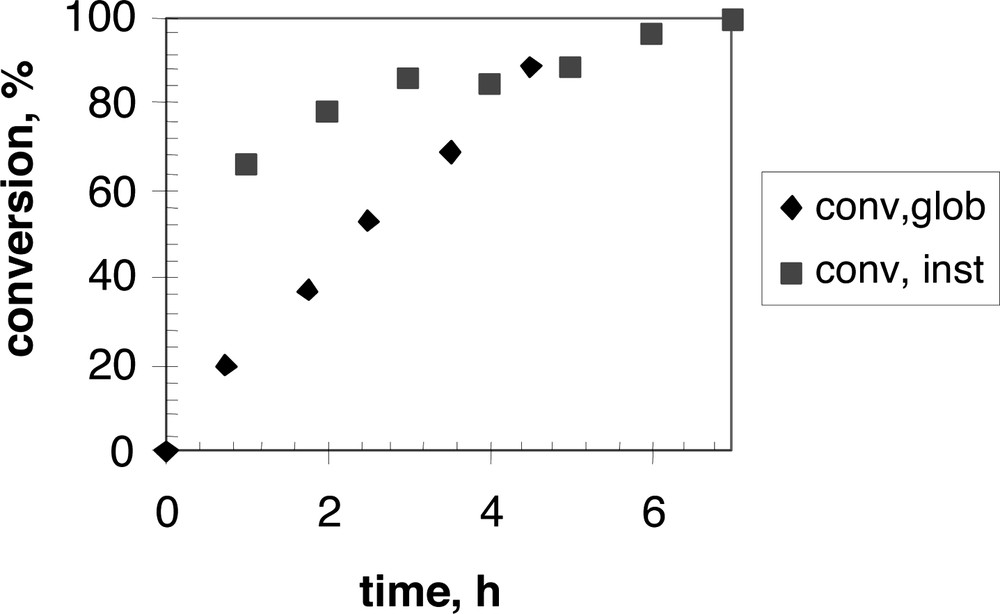
Core-shell seeded emulsion copolymerization of styrene/butylacrylate (1:1) using the zwitterionic surfmer 7a: conversions at 70 °C (instantaneous and global) versus time.
3.5 Latex stability
All the latexes remain stable upon addition of 0.1 N solutions of NaCl, but floculate if higher concentrations of electrolytes are used. They also resist to the addition of ethanol. However freeze-thawing tests cause floculation in all cases. In our previous work [9], using derivatives of hemiesters, similar results were obtained, except in one case in which a zwitterionic surfmer was shown to pass successfully the freeze-thawing test.
4 Conclusion
A series of polymerizable surfactants derived from the reaction of maleic isoimides containing a hydrophobic group (alkyl or benzyl) have been prepared. Maleic isoimides can be regarded as activated forms of maleic acid hemiamides useful for conversion of the last into maleic diamides – polymerizable surfactants (surfmers). Application of amines with different hydrophilicity allows varying the ratio between hydrophilic and hydrophobic parts of the surfmers. Hydrophilicity of maleic diamides can be further amplified by alkylation of external amino groups of the aminoalkyl diamides with alkyl halides, alkyl tosylates or 1,3-propane sultone. They belong to the classes of non-ionic, cationic or zwitterionic surfactants. Selection of them is determined by their colloidal properties, in view to be engaged in emulsion polymerization experiments. Their CMC is within the usual range of many usual surfactants. When the hydrophobic group is a long-chain alkyl group, good results are obtained, as well in batch polymerization of styrene, as in core-shell-seeded semi-batch copolymerization of styrene and butyl acrylate. Almost complete conversions are obtained in convenient times (few hours), with production of rather monodisperse particles of diameter in the range 80–200 nm, and very few, if any, coagulum. In batch polymerization of styrene, all the surfmers are either grafted or strongly adsorbed onto the particle surface. This adsorption is somewhat more limited in the case of core-shell copolymers, because the very surface of the corresponding particles is believed to include chiefly butyl acrylate units. The resistance of all these latexes to the main stability tests is rather disappointing.
Acknowledgements
This work was supported chiefly by the European Commission (Contract BRPR CT 97 0525). Some help was also given also by the French Ministry of Foreign Affairs (Embassy of France in Latvia).


