1 Introduction
FAMEs (Fatty Acid Methyl Esters), which are obtained by transesterification of natural triglycerides [1,2], are key reactants of a lot of important process in the chemical industry being employed to get a wide range of products used different industrial fields. The main products manufactured by FAMEs are long chain carboxylic acids, detergents, mono and di-glycerides, methyl esters of fatty acids, food additives, cosmetics, pharmaceuticals and alternative diesel oils (Bio Diesel) [3–5].
Several process are normally employed for homogeneous catalytic transesterification, but these reactions suffer from the presence of by-products that reduce selectivity and increase the separation time between the products, FAMEs and glycerine dramatically. If the reaction is carried out under microwaves, separation time is very short and the quantity of by-products is reduced, though not negligible. In previous works [6–8], it has been proven that microwaves are more effective than traditional heating in activating transesterification reactions by means of homogeneous catalysis (alcoholysis, glycerolysis), as they allow a reduction of the reaction time and temperatures under the same operating conditions.
The use of heterogeneous catalysts such as ZnAl2O4 + Al2O3 + ZnO [9] allows a drastic reduction of by-products and shortens their separation time too; on the other hand, traditional heating requires higher temperatures and a longer reaction time, thus increasing energy consumption.
Microwaves have been employed in this work as a well-proven method to activate the transesterification reaction of triglycerides with methyl esters using heterogeneous catalysts.
The reactions have been carried out with different heterogeneous catalysts, by studying the progression of the reaction time, the effect of the oil-methyl ester molar ratio and the catalyst amount.
Reactions have also been carried out with traditional heating to allow comparison with microwave effects.
2 Experimental
2.1 Reagents and catalysts
At first, preliminary tests of transesterification have been carried out with rapeseed oil, provided by Cognis S.p.A., whose composition is reported in Table 1, and with heterogeneous catalysts reported in Table 2. KSF montmorillonite, K10 montmorillonite, tin oxide, sodium carbonate catalysts have been purchased from Aldrich, while the 3 A zeolite has been provided by the Union Carbide Company. The K2FeO4-based catalyst has been prepared according to literature report [10], while the ZnO catalyst supported on Al2O3 has been prepared via sol–gel route from zinc powder, aluminium sec-butyloxide and propionic acid.
Rapeseed oil composition
| Fatty acid with number of carbon | 16 | 18 | 18 = | 18 = = | 18 = = = | 22 = |
| (%) | 4.5 | < 2.5 | 58 | 21 | 9.5 | < 1.5 |
| Acidity number (mg KOH/g oil) | < 0.5 | |||||
| Iodine number (g J2/100 g oil) | 114 ± 6 | |||||
| Saponification number (mg KOH/g oil) | 190 ± 5 |
Catalytic conversion of rapeseed oil into FAMEs over heterogeneous catalysts in the presence of microwaves
| Catalyst | Reaction time (min) | Yield% FAMEs |
| None | 10 | 0 |
| 60 | 0.2 | |
| Montmorillonite KSF | 10 | 7 |
| 60 | 51 | |
| Montmorillonite K 10 | 10 | 2 |
| 60 | 10.2 | |
| Zeolite 3 A | 10 | 1.3 |
| 60 | 4 | |
| K2FeO4 supported on Fe2O3 (*) | 10 | 0 |
| 60 | 0 | |
| ZnO supported on Al2O3 (**) | 10 | 0.4 |
| 60 | 2.1 | |
| SnO | 10 | 0.3 |
| 60 | 0.8 | |
| Na2CO3 | 10 | 0 |
| 60 | 0 |
Tests under microwaves have particularly been conducted on montmorillonite, taking into account the effects of the oil:catalyst ratio, the oil:methyl ester ratio, and the contact time. Comparison tests with traditional heating have also been carried out.
2.2 Apparatus
Tests under microwave irradiation have been conducted with a Milestone Ethos 1600 oven working at 2.45 GHz, with a power up to 1000 W provided with a MPR 600/12 rotor.
A Buchi autoclave, heated by means of silicon oil, has been employed in all test performed with thermal heating under pressure.
2.3 Product separation and analysis
Once the reaction has been completed, the obtained products have been cooled at room temperature, then the excess of methanol has been stripped by bubbling dry nitrogen and finally two layers have been obtained: the top layer is essentially Bio Diesel (fatty acid methyl esters).
A specific analysis has been conducted to determine only the amount of FAMEs by means of GC HP5890.
The analysis conditions have been: injector splitless 90 °C, detector FID 350 °C, Helium as a carrier gas, oven 2 min 75 °C, 16 °C min–1 up to 140 °C, 4 °C min–1 up to 240 °C, 12 °C min–1 up to 300 °C, 5 min 300 °C, ISTD methyl myristate J.T. Baker > 99%, solvent n-hexane (Riedel de Haen > 99%).
The amount of esters has been determined according to CEN TC 307 WI 040 regulation, while the quantities of glycerol, mono-, di- and triglycerides have been calculated according to CEN TC 307 WI 042 regulation.
3 Results and discussion
Tests with heterogeneous catalysts have first been carried out as a general investigation (10 and 60 min), and then the most active catalysts have been selected and subjected to a longer reaction time (up to 9 h). The influence of the main parameters has been analysed only in the case of catalysts that have given the best performances.
3.1 Preliminary tests
Initially, the catalysts have been subjected to microwave irradiation at 170 °C for 10 min. The methyl alcohol:oil molar ratio is 9:1, ratio already used for tests of homogeneous catalysis. Afterwards tests have been conducted under the same conditions, but lengthening the reaction time to 60 min. All tests have been compared by means of reactions performed without any catalyst. The results expressed as yields in methyl esters are reported in Table 2.
This preliminary study has allowed the most active tested catalysts, that is to say the KSF montmotillonite, the K10 montmorillonite and Zeolite 3A to be selected. Samples relative to ferric-oxide-based catalysts have not been analysed, being made up of a syrupy mass that is not separable.
3.2 Investigation tests
The above-mentioned most active catalysts have been further studied. Reactions under the same operating conditions have been carried out in order to evaluate the progression of the methyl ester yield. Some tests have lasted 3, 5, 7 or 9 h (Fig. 1 ). Comparison tests between thermal activation and microwave irradiation have been performed in order to evaluate the effect of microwaves on the transesterification catalysed by the KSF Montmorillonite.
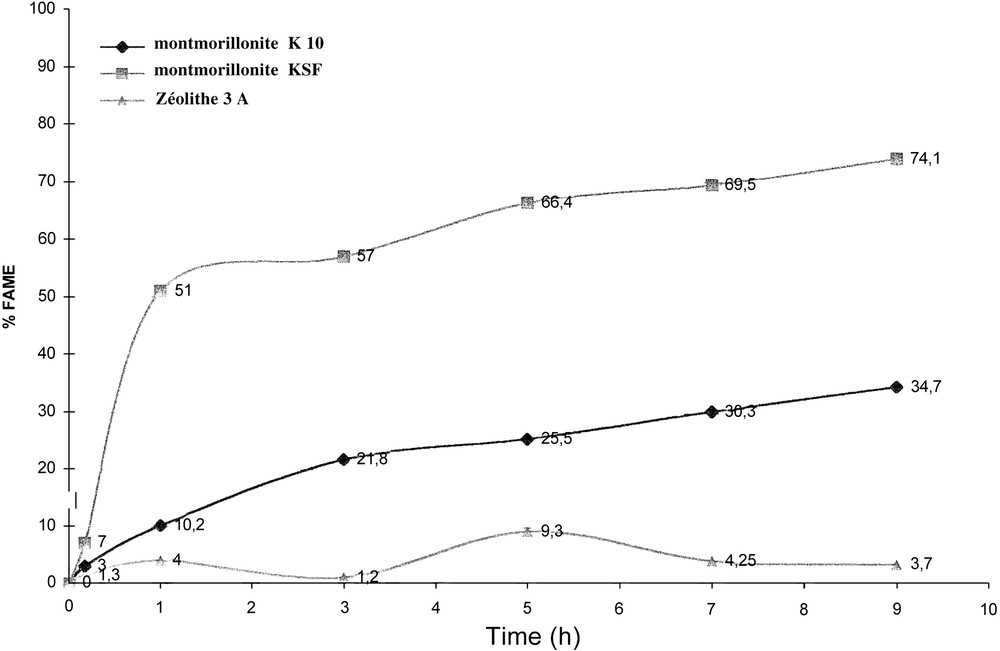
Methyl ester yield progression with reaction time over 3 A Zeolite and K10 or KSF montmorillonites.
4 Discussion
The results show that microwaves allow higher yields more quickly. The reaction temperature (zero time) is reached in 15 min by microwaves and in 75 min (35 to reach 140 °C and further 40 to pass from 140 °C to 170 °C) by thermal heating. For this reason, it has been necessary to take into account the yield in methyl esters once the reaction temperature has been reached.
Consequently, the zero time reported in Table 5 takes into account this difference, i.e. the yield is higher by means of traditional heating than by microwaves. Moreover, the different yield progression of the two heating methods process confirms that microwaves influence the physical steps of the reaction.
Microwave heating effect versus conventional heating on the conversion of oil to fatty methyl esters
| Heating mode | Time (min) | |||
| 0 (*) | 30 | 60 | 120 | |
| conventional | 22.3 | 30.3 | 32.1 | — |
| microwaves | — | 24.65 | 51 | 54 |
(*) Using microwave heating, the reaction temperature is reached after 15 min, after switching on the oven.
The KSF Montmorillonite has proven to be the most effective of the tested catalysts in this reaction performed under microwave irradiation. The results of this catalyst and the ones reported in the literature [9] show that the evolution of FAMEs and the effect of the methyl alcohol-oil ratio are comparable in both cases. Besides, after a conversion of the 50%, the rate of FAMEs formation makes slower in consequence of the kinetic evolution in a batch reactor. The KSF catalyst works at a lower temperature (170 °C) than the catalyst reported in the literature [9] (200 °C), with an almost equal methyl alcohol–oil ratio (Fig. 1).
The effects on the different methyl alcohol:oil ratio on the activity of KSF and K10 evaluated after 1-h reaction time are reported in Table 3. KSF gives a better yield in FAMEs (56.6 vs 51) with the 18:1 ratio; it gives the same results with a 36:1 ratio (50.5 vs 51). On the contrary, K10 shows a constant increase in the conversion into FAMEs in parallel with the methyl alcohol:oil ratio varying from 10.2% with 9:1 ratio to 12.3% with the 18:1 ratio to reach 13.5% with 36:1.
Effect of methanol/oil ratio on the conversion of oil to fatty methyl esters
| Catalyst | Methanol:oil molar ratio | ||
| 9:1 | 18:1 | 36:1 | |
| KSF | 51.00 | 56.65 | 50.54 |
| K 10 | 10.20 | 12.30 | 13.50 |
The influence of the catalyst percentage has been taken into account, as reported in Table 4, at different times only in the case of KSF catalysts. The increase in the catalyst percentage has no advantage; on the contrary, the reaction recedes.
Effect of oil/catalyst ratio (w/w) on the conversion of oil to fatty methyl esters
| Catalyst% (w/w) | Time (min) | ||
| 30 | 60 | 120 | |
| 10 | 24.6 | 51.0 | 54.0 |
| 50 | 25.6 | 32.3 | 7.4 |
Finally, comparing the tests conducted with KSF catalysts either by means of traditional thermal heating or by microwaves (Table 5), the effectiveness of the latter in activating transesterification reactions has been confirmed.
After 60 min under MW irradiation, the yield of FAMEs is 51% versus the 32% obtained by means of traditional heating.
The reason why KSF has proven to be the most interesting of the tested heterogeneous catalysts seems to be due to its high degree of acidity calculated by the Hammett acidity function (Table 6) and ranging between H2SO4 and HNO3.
Hammett acidity of the catalysts
| Catalysts | H0 (Hammett function) |
| Montmorillonite K 10 | –3 |
| Montmorillonite KSF | –8.2 |
| Sulphuric acid | –10 |
| Nitric acid | –5 |
Also K10 is fairly active and its degree of acidity is not so high. Other authors [11] have found an analogous advantage in esterification tests of stearic acid with butanol performed by the same catalytic system.
It is reasonable to think that the physical steps present in this reaction, such as the reactant immiscibility and the possible problems of mass transfer due to the presence of a heterogeneous catalyst, may properly be quickened by microwaves.
As for the separation of glycerol, we have observed a partial degradation of this product mainly with KSF catalyst (FTIR analysis).
5 Conclusions
Microwave irradiation has been revealed as faster than thermal heating for the synthesis of methyl esters (BioDiesel) from triglycerides in the presence of heterogeneous catalysts.
The KSF and the K10 montmorillonite have turned out be the most promising of the tested heterogeneous catalysts.
The results obtained with KSF montmorillonite under microwaves are important and the utilisation of this promising catalyst could be proposed for a continuous process.
Acknowledgements
This work was supported by Cofin-Miur 2001/2003.


