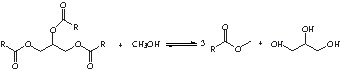1 Introduction
Numerous products may be produced by using raw materials such as vegetable oils. The renewability of the raw materials and the specific properties of these compounds are of growing interest for the industry [1].
The main reactions used first in oleochemistry were the hydrolysis or the methanolysis of triglycerides leading to glycerol and fatty acids or fatty methyl esters.
However, as these reactions also lead to the formation of glycerol, we have to find valuable applications for this side-product. The glycerol transformation into glycerol monoesters has significant applications in food, pharmaceutical, cosmetics or in detergent chemistry [2].
In a previous paper [3], we have shown that basic solids such as magnesium or cerium oxide could be used as efficient catalysts for this reaction. In varying the preparation method of these oxides, we were able to increase the activity so that the reaction rate was similar to that obtained in the presence of homogeneous catalysts. However, the monoglycerides selectivity was rather similar and a distribution of mono/di/tri glycerides close to 50%/40%/10% was observed.
In order to increase the monoglycerides selectivity, we suggested a dispersion of active oxides at the surface of an MCM-41 type mesoporous structure.
MCM-41 are silica or silico-alumina characterized by a regular hexagonal arrangement of cylindrical pores. The pore diameter is higher than in the case of zeolites (typically 3 nm or more) with a specific surface area of more than 1000 m2 g–1 [4,5]. Moreover, several works gave a strong indication of a quite significant thermal stability [6].
Several attempts were already done to obtain monoglycerides in using mesoporous catalysts. For example, Jacobs et al. used –SO3H groups grafted over MCM-41 to catalyse glycerol esterification [7]. This method was recently improved by grafting alkyl and sulfonates groups in order to tune the hydrophilicity of the catalyst [8,9]. However, that processes involved acid catalysts that are well known to induce glycerol dehydration and thus side-products generation. Brunel et al. used MCM-41 functionalised with amines to perform glycidol esterification. As that reagent has only one hydroxyl function, monoglycerides, which are the only products that can be formed during esterification, are obtained with a high yield [10].
In this paper, several acid methyl esters [lauric (dodecanoic, C11H23CO2CH3), myristic (tetradecanoic, C13H27CO2CH3) and stearic (octadecanoic, C17H35CO2CH3) acid methyl esters] were used in the glycerol transesterification reaction over several magnesium containing MCM-41 and the influence of some structural properties of the solids over the shape selectivity will be discussed.
2 Experimental
2.1 Synthesis of the catalyst
2.1.1 Synthesis of the MCM-41 support
This synthesis is the result of several works done in the laboratory [11,12]. A mixture of 1.47 g NaOH (37 mmol) and 13.04 g hexadecyltrimethylammonium bromide (9.1 mmol) in 200 ml water was heated at 40 °C in order to dissolve the surfactant. For silico-alumina MCM-41, 2.43 g Al(NO3)39 H2O (8 mmol) were added. A little amount of antifoaming agent (Antifoam A, Sigma) was also added in order to improve the synthesis. The pH was stabilized at 10.5 with nitric acid (1 M), and 32.04 g of a 30% solution of Na2Si3O7 (40 mmol) were slowly added, while maintaining the pH to 10.5. The resulting gel was heated in a sealed Teflon reactor during 16 h at 100 °C without stirring. The content was then filtrated, dried and finally calcinated at 550 °C (after a temperature increase of 1 °C min–1) during 16 h. The support is called AlMCM-41 if containing alumina or MCM-41 without alumina.
2.1.2 Synthesis of the Mg-containing catalyst
The method is given for Si/Mg = 4, and for another Mg stoichiometry, the content of precursors must be adjusted.
A mixture containing 3 g of MCM-41 freshly calcinated, 2.96 g Mg(CH3CO2)24 H2O and 50 g MeOH was stirred at room temperature during 2 h. The solvent was then evaporated. The solid was then calcinated at 450 °C (after a temperature increase of 1 °C min–1) during 16 h.
These solids exhibit a specific surface area of about 800 m2 g–1 and are characterized by an hexagonal array of cylindrical pores, as shown by X-ray diffraction and Transmission Electronic Microscopy (Figs. 1 and 2 ).

XRD of Mg-AlMCM-41.
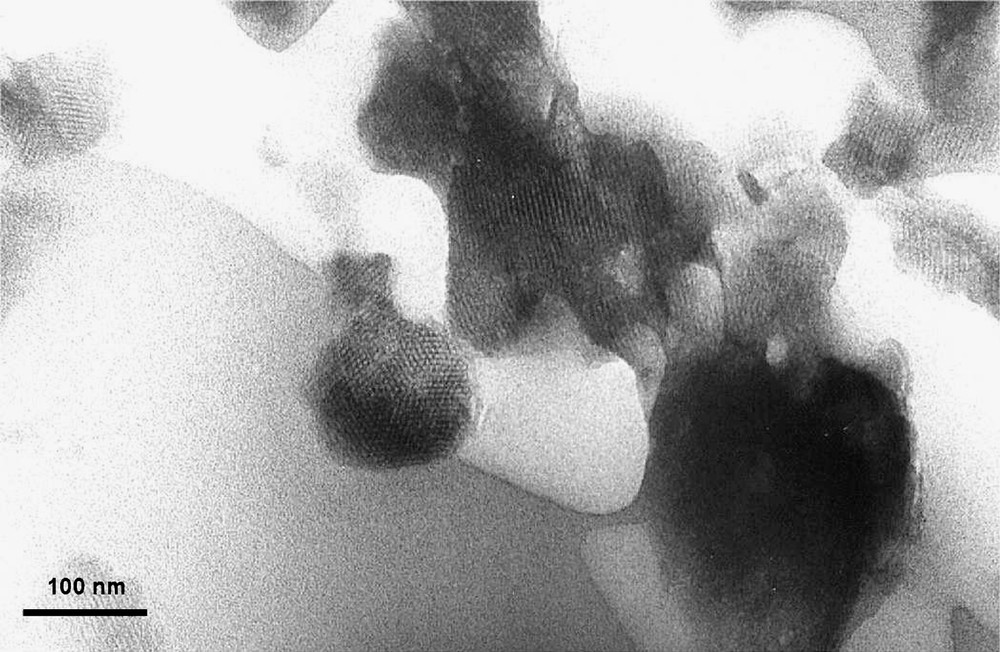
TEM of Mg-AlMCM-41.
The solids obtained are called Mg-AlMCM-41 or Mg-MCM-41, whether they contain alumina or not.
2.2 Catalytic test
2.2.1 Without preliminary impregnation of the ester
In a 100-ml three-neck flask, the glycerol and the methyl ester were mixed and heated to 220 °C under stirring (500 rpm) without solvent and under nitrogen atmosphere. When the temperature reached 220 °C, the catalyst was added, which corresponded to the starting time of the reaction. The amounts of reactants are given in Table 1.
Amount of reactants for the preparation of glycerol esters over mesoporous solids
| Gly/ester | m C12 (g) | m C14 (g) | m C18 (g) | m catalyst (g) | m glycerol (g) |
| [mol gly/mol ester] | |||||
| 1 | 9.9 | 11.2 | 14.0 | 0.5 | 4.5 |
| 3 | 7.5 | 8.46 | 10.38 | 0.55 | 10 |
2.2.2 With preliminary impregnation of the ester
In the same flask and experimental conditions, the methyl ester and the catalyst were mixed. After 2 h, the glycerol was added, which corresponded to the starting time of the reaction. The amounts of reactants are also given in Table 1.
2.3 Analysis of the products
The products were analysed with a Varian 3300 GPC equipped with a FID detector, an on-column injector and a BPX5 (SGE) column. Before analysis, the products are silylated to prevent their degradation according to the method described by Sahasrabuhde [13].
The reagent conversion to mono-, di-, and triglycerides is given by the following equation:
The selectivities to monoglycerides, diglycerides and triglycerides are given by the following equations:
3 Results
3.1 Reactivity of glycerol with various methyl esters over Mg-AlMCM-41 type catalysts
The reaction of lauric (C12), myristic (C14) and stearic (C18) acid methyl esters with glycerol in the presence of magnesium-containing silico-aluminate Mg-AlMCM-41 (glycerol / methyl ester ratio equal to 1 or 3) were performed without or with the pre-impregnation of the methyl ester.
3.1.1 Without methyl ester pre-impregnation
The results are completely different from that obtained with homogeneous catalysts [2] for which a monoglyceride selectivity of 40% was obtained at a conversion of 80% with a glycerol/methyl ester ratio of 1. In this study, the monoglyceride selectivity seems to be very low (respectively 13, 8 and 1%) at a conversion of 80%. In fact, this catalyst mainly gives glycerol diesters, which could be due to the strong hydrophilicity of the catalyst surface. Glycerol was strongly adsorbed and filled in the pores of the MCM-41 structure, whereas the hydrophobic methyl ester was mainly outside the pores where the reaction occurred. As the MCM-41 pore volume is important (about 1 cm3 g–1), a significant amount of glycerol is unavailable and then the apparent glycerol/methyl ester ratio is far less than 1. Indeed, if we assume a pore volume of 1.2 cm3 g–1, a glycerol density of 1.216, a quick calculation gives a ‘loss’ of glycerol of about 18%.
3.1.2 With methyl ester pre-impregnation
In order to confirm such hypothesis, we did the same experiments with a preliminary impregnation of the methyl ester and the results shown in Table 2 are totally different. Indeed, from the two shortest methyl esters (C12 and C14), the monoglycerides selectivity is higher than that obtained with homogeneous catalysts, which clearly suggests a strong adsorption of glycerol.
Transesterification of glycerol over Mg-AlMCM-41 after a preliminary ester impregnation
| Ester | Gl/ester | Time to 50% (h)a | Selectivity at 50%b | Time to 80% (h)a | Selectivity at 80%b | Initial activity (mmol h–1 g–1) |
| C12 | 1 | 2 | 65 | 4 | 45 | 2.17 |
| C14 | 1 | 7 | 60 | 13 | 55 | 0.87 |
| C18 | 1 | 14 | 28 | 26 | 15 | 0.64 |
| C12 | 3 | 2 | 80 | 3 | 70 | 2.18 |
| C14 | 3 | 8 | 75 | 10 | 68 | 0.94 |
| C18 | 3 | 7 | 70 | 10 | 40 | 0.55 |
By doing first an impregnation of the catalyst with the methyl ester, the surface coverage of the catalyst with the ester was increased. At the beginning of the reaction, the glycerol, which is more labile and far more hydrophilic than the methyl esters, migrated into the pores where the reaction took place with the methyl esters. When the glycerol fully replaced the methyl ester in the pores, the reaction ending rate and the selectivity were similar to that obtained in the previous case.
The comparison of the results obtained with an excess of glycerol (Tables 2 and 3) indicated as expected an increase of glycerol monoesters selectivity, particularly with methyl dodecanoate or tetradecanoate. It was a first difference suggesting that the porous structure was more or less without effect when methyl octadecanoate was the reagent instead of ‘shortest’ esters. The reaction rates also increased when a glycerol excess was used in a normal experiment (Table 3). On the other hand, there was no change when the catalyst was pre-impregnated with the methyl ester, which was a strong indication of a low coverage of the catalyst surface with the methyl ester.
Catalytic transesterification of glycerol over Mg-AlMCM-41 without preliminary impregnation of the ester
| Ester | Gly/ester | Time to 50% (h)a | Selectivity at 50%b | Time to 80% (h)a | Selectivity at 80%b | Initial activity (mmol h–1 g–1) |
| C12 | 1 | 11 | 45 | 20 | 13 | 0.54 |
| C14 | 1 | 19 | 17.5 | 33 | 8 | 0.44 |
| C18 | 1 | 18 | 10.5 | 40 | 1 | 0.31 |
| C12 | 3 | 2 | 75 | 3 | 70 | 2.12 |
| C14 | 3 | 9 | 65 | 14 | 62 | 0.92 |
| C18 | 3 | 10 | 55 | 13 | 50 | 0.54 |
a Time needed to reach a 50% (or 80%) methyl ester conversion.
b Monoglycerides selectivity at 50% (or 80%) methyl ester conversion.
3.2 Mg-MCM-41 type catalysts
In order to improve the monoglyceride selectivity, less hydrophilic supports like pure silica MCM-41 were synthesized. The Mg-containing pure silica Mg-MCM-41 was then prepared and dehydrated (700 °C, 16 h at reduced pressure) in order to transform surface Si–OH groups into more hydrophobic Si–O–Si groups. Then the catalytic reaction was performed with methyl octadodecanoate in the presence of both catalysts and the results were compared to that of Mg-AlMCM41.
An increase in both selectivity and activity with a decrease in hydrophilicity was observed (Fig. 3 ), the most important changes being consecutive to the absence of alumina (the hydroxyls groups eliminated from dehydration of the solid seems out of interest for the adsorption and/or the reaction). The glycerol is less adsorbed over the surface compared to the methyl ester and the selectivity remains more important than that observed with Mg-AlMCM-41 (Fig. 3).

Transesterification of glycerol with methyl octadocanoate (gly/C18 = 1) in the presence of Mg-MCM-41 and Mg-AlMCM41 catalysts.
3.3 Pore-size effect
The results presented in the previous tables and figures indicated that the highest selectivities to glycerol monoesters are obtained either with the lauric or the myristic acid methyl ester. Such selectivity modifications could be due to changes in the hydrophobicity of the catalyst or/and of the hydrocarbon chain length of the ester. Indeed, the hydrophilicity of the methyl ester decreases as the chain length increases, so that a decrease in the monoglycerides selectivity with the chain length of the methyl ester should be expected. In Tables 2 and 3, such variation is observed, but mainly with the C18-ester.
In order to check the effect of the pore size of the catalysts, Mg-MCM41 materials with a smaller pore size were synthesized in using smaller surfactants such as tetradecyltrimethylammonium bromide (C14H29N(CH3)3Br) and dodecyltrimethylammonium bromide (C12H25N(CH3)3Br) instead of the hexadecyltrimethylammonium bromide (C16H33N(CH3)3Br). The interreticular distance d100 of the mesophases varied from 3.4 to 4.1 nm and the surface area was higher than 1000 m2 g–1 (Table 4).
d100 of the ‘smaller pore size’ mesophases
| Surfactant used | C12 | C14 | C16 |
| d100 (nm) | 3.43 | 3.65 | 4.12 |
These catalysts were tested in the glycerol transesterification with the methyl laurate, in the following experimental conditions; a temperature of 220 °C and a ratio glycerol/ester of 2 (more representative of real conditions).
It appears (Fig. 4 ) that the decrease in the pore size did not change the initial activity. A sharper increase of the conversion was only observed over the catalyst with the largest pores after 3 h of reaction, which could be the result of an increasing effect of the diffusion processes with the diminution of the pores size. Indeed, as the pores aperture was reduced, molecular diffusion increases while Knudsen diffusion is also more important. However, if the monoglyceride selectivities obtained with ‘C14 and C16 catalysts’ were very similar, the selectivity obtained in the presence of ‘C12 catalysts’ was higher. This result clearly shows that the pore size reduction has a greater impact over the molecular diffusion control inside the pores of the catalyst. Decreasing the pore size avoids polyesterification of glycerol, so that selectivity and yield to monoesters of 75% are obtained even if the ratio glycerol/methylester is much lower than in the previous cases (Tables 2 and 3).
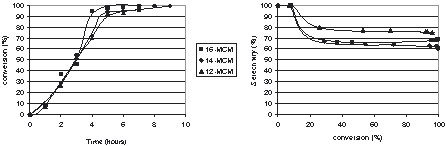
Glycerol transesterification with methyl laurate over of ‘smaller pore size’ mesoporous Mg-MCM41 catalysts. T = 220 °C, glycerol/ester = 2.
At this stage, it must be also added that the activity and the selectivity to monoesterification are not so important if the mesoporous structure of the catalyst is not as well defined and organized as above. Indeed, if the preparation procedure is modified so as to obtain a more amorphous solid with a larger pore size distribution (even centred around the same mean value of 3.5 nm), there appears an increase in both diesters and triesters formation. Then besides the hydrophilicity effect, there is also a strong influence of the pore size of the catalyst over the selectivity.
4 Conclusion
This study clearly shows that porous solids such as MCM-41 promoted with magnesium species can be efficient catalysts for clean synthesis processes, particularly when the synthesis of the porous material and the experimental conditions are finely tuned to the reagents. Here glycerol monoesters were synthesized with a yield up to 80%, which is quite higher than the 40% monoglycerides obtained with homogeneous catalysts. In our knowledge, it is the first time that such a shape selectivity is observed with mesoporous catalysts for such reactions.
Acknowledgements
The authors want to thank the Stearinerie Dubois and ADEME–AGRICE for fruitful discussions and financial support.