Carbohydrates are the most abundant natural substances produced by living organisms, especially plants. They are therefore the main products of agriculture, however only a small part of them is really used and the major application is obviously nutrition and food. Non-food applications of carbohydrates [1] would offer tremendous opportunities to agriculture and also environmentally safe alternatives to the classical chemistry based on fossil sources [2,3].
One of the main developments of carbohydrates towards non-food applications lies on the synthesis of new and green surfactants [1,4]. Indeed, carbohydrates fulfil half of the required properties for a surfactant with their hydrophilic structure. Grafting long hydrophobic chain onto them would yield a compound having tensioactive properties. Few examples have already appeared in the literature [4,5]. Surprisingly, aldonolactones have almost never been studied for this purpose [6,7], although they could lead to non-ionic as well as ionic detergents.
In this communication, we present a mild and efficient one-pot access to tensioactives compounds derived from aldonolactones using either chemical reagents or enzymes.
d-Glucono-1,5-lactone 1a and l-galactono-1,4-lactone 2 were selected for their availability and submitted to various protocols introducing an alkyl chain on them. To get authentic samples, we first relied on a classical protection–deprotection sequence allowing to unambiguously preparing aldonolactones carrying only one chain but at different positions. Using selective and successive protections, any acylated derivatives of 1a,b or 2 can be produced after a tedious multistep sequence [8]. However, industrial applications and developments would require a synthesis as short as possible, we thus looked for a selective and rapid method able to introduce an alkyl chain. Plusquellec type reagents are known to provide selective acylation of carbohydrates [9], we thus applied these reagents to the lactones 1a and 2 in the presence of bases in various solvents (Scheme 1 ).

To find the right conditions, N-(11-undecenoyl)-1,3-thiazolidine-2-thione 3 (R = (CH2)8CH=CH2 in Scheme 1) [10] was first used as a model. In this reagent, the alkyl chain was selected with a chain length long enough to induce tensioactive properties and with a double bond that can act as a reporter group, facilitating NMR studies of the products formed. The treatment of 1a or 2 with N-(11-undecenoyl)-1,3-thiazolidine-2-thione 3 in appropriate conditions indeed provided the expected monoacylated products. Typical results were reported in Table 1.
Chemical acylation of aldonolactones with reagent 3.
a Yields are given for pure isolated products.
b R = (CH2)8CH=CH2.
c Py = pyridine.
d Various amines were experimented and NEt3 proved to be the most convenient.
Strong bases proved to be inefficient and no acylation product could be detected under these conditions (entries 1, 3, 5). The starting lactones were recovered untouched, except for d-glucono-1,5-lactone 1a, which was quantitatively isomerised to the corresponding 1,4-lactone 1b (entry 1). Mild bases such as amines were however effective, especially when DMF was used as solvent (entries 2, 4, 6).
The acylation position proved to be highly dependent on the starting lactone structure. d-Gluconolactone as a 1,5-lactone 1a or as a 1,4-lactone 1b gave only the 6-undecenoyl ester as expected due to the primary position of the 6-hydroxyl group (entries 2, 4), but the l-galactono-1,4-lactone 2 gave a product where the acyl chain is located at the 2 position (entry 6). Control experiments have shown that this selectivity was not the result of acyl migration. Conformational analysis revealed that the hydroxyl group in the 2-position of 1a,b and 2 has different environment. Further work is in progress to elucidate this reactivity difference.
Although highly selective, this direct acylation method is not really practical, since the yield of acylated product remained around 20% whatever the solvent and the base used. The yields could be increased using other acylating reagents, the selectivity however decreased depending on both aldonolactone and reagent structures. It is nevertheless worth to point out that these reactions provide in a one-pot process only one acylated compound, which can easily be recovered by chromatography from the remaining starting materials, which itself could be recycled.
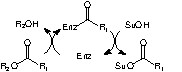
Enzymes can also realize the selective acylation of various hydroxylated compounds, including carbohydrates, providing water is excluded from the reaction medium [11]. The enzymes used are hydrolytic enzymes, which are first acylated by an appropriate reagent. In the absence of water, the so-formed acyl enzyme intermediate can be attacked by a hydroxyl group of the substrate. The enzyme is thus regenerated and the hydroxyl group acylated (Scheme 2 ).
The application of this process to aldonolactones could however raise several problems. Indeed, the lactone group in these carbohydrates could act as an acylating agent and thus polymerisation could occur as shown in Scheme 3 .
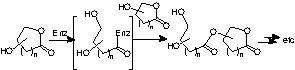
An industrial group already reported an enzymatic acylation of gluconolactone, but they indeed obtained mixture of compounds [6]. Nevertheless, we submitted 1 and 2 to an enzyme screening from which the crude extract of pig pancreas (PPL) and Candida antartica lipase (CAL) emerged. All other enzymes leaved the starting aldonolactones untouched, no oligo- or polymerisation product have been detected. Since PPL and CAL were able to catalyse the acylation of d-glucono-1,5-lactone, conditions’ optimisation was then conducted with each enzyme, but the most interesting results were obtained with CAL and they are collected in Table 2.
Enzymatic acylation of aldonolactones.a
a The reactions were performed in tBuOH at 45 °C in the presence of molecular sieves to remove water and/or alcohol.
b 8 equiv of the acylating reagent were used TFE = –CH2CF3.
c Yields are given for pure isolated products; 6b, 7, R = –(CH2)16CH3; 4b, 8, R = –(CH2)8CH=CH2.
In sharp contrast with the chemical route, only the 6-acylated compounds were isolated whatever the structure of the starting lactones and moreover, the reaction could give excellent yields. Yields do not seem to be correlated to the chain length (entries 2 vs 3 and 6 vs 7) but rather to the acylating power of the reagent used. Indeed, the more electron-withdrawing the ester, the more efficient the acylation (entries 4 vs 3 and 8 vs 7). Although acids have been reported as acylating agents in enzymatic reactions [12], they proved to be unreactive here (entries 1, 5). Ethyl esters were not reactive enough (entries 2, 3, 6, 7), they probably cannot efficiently form the acyl enzyme intermediates (see Scheme 2, R2 = Et), even after prolonged reaction time. With the 2,2,2-trifluoroethyl ester, the acyl enzyme is probably easily formed by displacement of the labile 2,2,2-trifluoroethanol (see Scheme 2, R2 = CF3CH2), the reaction is thus more rapid and much more efficient (entries 4, 8).
The mechanism of this enzymatic reaction implies that the carbohydrate must react with the acyl enzyme intermediate. The carbohydrate must thus enter the enzyme up to its active site and one of carbohydrate hydroxyl groups should displaced the acyl group linked to the enzyme active site. This complex mechanism could explain the very high selectivity observed for acylation of the primary hydroxyl group, independently of the lactone structure.
The tensioactives properties (CMC, emulsifying properties and foam formation) of the compounds obtained either from the chemical route or from the enzymatic step have been investigated. These physicochemical studies will be described later. Some of the compounds exhibited interesting properties, which could lead to industrial applications as mild detergents or emulsifiers.
In conclusion, we have been able to selectively acylate aldonolactones in a one-pot process using either chemical reagents or enzymes. The so formed derivatives exhibited interesting tensioactive properties. Aldonolactones were thus converted to green tensioactives compounds.
Acknowledgements
This paper is dedicated to the memory of Prof. J. Chuche, ‘Université de Reims-Champagne-Ardenne’. The company ARD (Pomacle) is gratefully acknowledged for generous gifts of aldonolactones and for performing the tensioactive evaluation of our products. The authors thank the ‘Région Champagne-Ardenne’ for financial supports.


