1 Introduction
Dendrimers are synthesized by the use of multistep iterative controlled reaction procedures, whereas hyperbranched polymers are prepared by one pot reaction that results in a less regularly unified structural conformation, accompanied by broad molecular weight distributions [1–5]. Recently, dendrimers have attracted considerable attention due to their new properties caused by their unique structural architecture and useful abilities for new material science [6–9]. However, the preparation of well-characterized and highly branched dendritic macromolecules required a time-spending process [10–12]. On the other hand, hyperbranched polymers can be prepared by one-step bulk reaction as a general polymerization process [13]. Hence, the product had an uncontrolled structural conformation containing the average number of unreacted functional groups according to the molecular size everywhere on the molecular surface and inside. The remained functional groups such as double bonds in the hyperbranched polymer could be successfully used as the core of a new type dendrimer [14–15].
The resulting products from the dendritic generation using the hyperbranched core could reveal less regular molecular distribution than the iterative generated general dendrimer, but the prepared dendrimer using hyperbranched core polymer had a molecular group with a different size, which have more functionalities than the core. The hyperbranched polymer with triallylsilane as a general model was prepared without solvents at high temperature and singly identified [16]. However, the hyperbranched polymer with Si–O bonds sourced from triallyloxysilane was not found in published article. In this paper, we wish to report on the synthetic method of a new hyperbranched polymer with triallyloxysilane, and the resulting polymer could be hopefully used as the core of the new type of dendritic molecules.
2 Results and discussion
Triallyloxysilane for the preparation of carbosiloxane hyperbranched polymers was prepared at –78 °C with three equivalents of allylalcohol and one equivalent of trichlorosilane in toluene containing TMEDA at the low temperature. The reaction product revealed very pure and obtained high yield. However, the reaction product was obtained as the mixture of triallyloxysilane and tetraoxyallylsilane at room temperature.
The AB3-type hyperbranched carbosiloxane polymer could be prepared from triallyloxysilane (H–Si(OCH2CH=CH2)3 under catalytic mild conditions at room temperature, which was progressed to a colorless polymer with a difficult running liquid type. The 1H NMR spectrum viewed the hyperbranched polymer confirmed by the disappearance of the Si-H signal of triallyloxysilane at 3.71 ppm and saw the new signals of the propylene group between 0.51 and 1.81 ppm by the cost of breaking double bonds in monomers. The AB3-G0 type hyperbranched polymer had two times of unreacted allyloxy groups on the polymerized branch. By 13C NMR spectroscopic views, the unreacted allyloxy groups were observed at 113.5–113.7, 133.5–134.7 ppm (Fig. 1). The GPC measurement of hyperbranched polymers (AB3-G0) led to the extremely broad molecular weight distribution of polydispersity index values (PDI; 3.66) at the retention time, which means the hyperbranched carbosilane was constructed by different molecular sizes. The MALDI TOF mass spectrum of AB3-G0 observed a series of molecular ions with a mass increment of 200 amu as repeating units of triallyloxysilane moieties at m/z = 823, 1023, 1223, etc. So, the mixed hyperbranched polymer increased with the monomer.
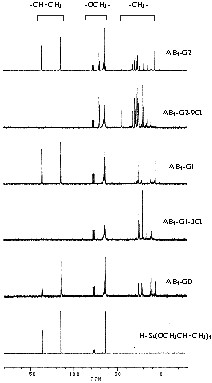
13C NMR spectra of H–Si(OCH2CH=CH2)3 and AB3-G0 to AB3-G2.
The reaction of the AB3-G0 with trichlorosilane in the presence of H2PtCl6 provided broken double bonds and increased SiCl3 groups on the polymer (Fig. 1). The hydrosilation of the hyperbranched polymer could be carried out at the RT. The NMR spectroscopic view of the hyperbranched polymer gave a very simple construction that observed the disappearance of allyl groups at 115 and 136 ppm and the appearance of many signals at sp3 area (Fig. 1). The hydrosilation of the hyperbranched polymer with trichlorosilane provided the complete disappearance of allyl groups on the polymer backbone. The continual alcoholysis reaction with allylalcohol was progressed to the first generation AB3-G1, which had three times of functional groups compared with the core polymer (Scheme 1). By GPC, the polydispersity of AB3-G1 had an almost unchanged value (PDI: 3.86) from the core polymer (PDI: 3.66). This means the conformational distribution of the growing polymer did not contain side reaction such as the copolymerization of cores. Therefore, the hyperbranched polymer could be used as the core for dendrimers. The MALDI TOF spectrum (Fig. 2) of AB3-G1 observed very complex and regularly observed signals. By the use of the same synthetic procedures such as hydrosilation with trichlorosilane and alcoholysis with allylalcohol, we produced the second generation AB3-G2 that had nine times of more functionalities than cores. The MALDI TOF spectrum (Fig. 2) of AB3-G2 observed a series of molecular ions with a mass increment of 200 amu as repeating units of Si(OCH2CH=CH2)3 moieties at m/z 679, 879, 1079, 1279, 1480, 1681, etc. which was the same evidence of AB3-G1 (Fig. 2).
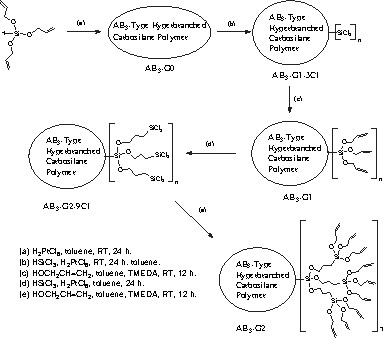
Schematic view of the dendronizing process of a hyperbranched polymer.
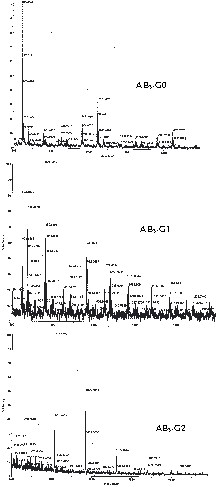
MALDI mass spectrum of AB3-G0 (top), AB3-G1 (center) and AB3-G2 (bottom).
In conclusion, we have shown that the preparation of hyperbranched polymers with triallyloxysilane at room temperature can be achieved via one simple-step platinum-catalyzed protocol. The prepared polymer can be used as the core of a carbosilane dendrimer.
3 Experimental
All reactions were carried out under a dried N2 atmosphere and THF was dried by sodium-benzophenone ketyl, while toluene was distilled from sodium metal. The NMR spectra were recorded on a Bruker AC-200 Spectrometer. With THF as a solvent for GPC, all GPC data were referred to polystyrene standards, and a combination of columns 105, 104, and 103 was employed. The Busan and Daejeon Branches of the Korean Basic Science Institute (KBSI) performed MALDI mass and elemental analysis.
3.1 Preparation of triallyloxysilane (H–Si(OCH2CH=CH2)3)
8.0 g (59.06 mmol; 5.96 ml) of fresh distilled trichlorosilane were dissolved in 200 ml of pentane and cooled to –78 °C and slowly added to 10.43 g (179.68 mmol) of allylalcohol. The reaction mixture was stirred 10 min at this temperature and continually slowly added to 6.80 g (58.51 mmol) of TMEDA and stirred for 1 h at this temperature. The product was isolated from the white salt by filtration and distilled the fine product at room temperature by vacuum. Yield: 7.41 g (37.00 mmol, 62%) of a colorless liquid. 1H NMR (ppm, CDCl3): δ = 4.17 (s, Si–H), 4.33 (s, OCH2), 5.02–5.40 (m, CH2=), 5.82–6.00 (m, CH=). 13C NMR (ppm, CDCl3): δ = 63.48 (OCH2), 114.98 (CH2=), 135.91 (CH=). MS: 199 (M-H)+, 158 (M–CH2CHCH2)+, 142 (M–OCH2CHCH2)+, 101 (SiO2CH2CH=CH2)+.
3.2 Preparation of AB3-G0
4.60 g (23.00 mmol) of triallyloxysilane and 0.06 g of H2PtCl6 were dissolved in 25 ml of toluene and stirred 24 h at the RT. Yield: 4.10 g (89%) of a light yellow liquid. 1H NMR (ppm, CDCl3): δ = 0.51–1.81 (m, CH2), 3.6–4.2 (m, OCH2), 4.30 (m, OCH2), 5.0–5.4 (m, CH2=), 5.91 (m, CH=). 13C NMR (ppm, CDCl3): δ = 6.19–25.65 (CH2), 63.48–66.21 (OCH2), 114.23–114.89 (CH2=), 136.12–136.67 (CH=).
3.3 Preparation of AB3-G1-3Cl
10.74 g (79.27mmol) of HSiCl3, added to 4.0 g of G0, were dissolved in 15 ml of toluene and continually added to 0.04 g of H2PtCl6 and stirred at the RT for 1 day. Yield: 9.16 g of a light yellow gel. The increasing rate of G0 was 5.16 g, which means 38.09 mmol of trichlorosilane was added to the core molecule. 1H NMR (ppm, CDCl3) δ = 0.80–2.20 (m, CH2), 3.85 (s, OCH2), 5.50~6.02 (m, CH=CH2). 13C NMR (ppm, CDCl3): δ = 10.90–25.18 (CH2), 63.52–65.24 (OCH2), 76.36–77.62.
3.4 Preparation of the first generation (AB3-G1)
9.16 g of G1P (increasing rate: 5.16 g; 38.09 mmol of trichlorosilane) were dissolved in 200 ml of toluene and added slowly to 8.2 g (141.26 mmol) of allylalcohol and cooled the reaction mixture. Continually added 6.64 g (57.09 mmol) of TMEDA to the cooled reaction mixture. The reaction mixture was warmed at the RT and stirred the reaction mixture for 1 day. The reaction product was purified by chromatography on a silica gel with THF as an eluent. Yield: 10.07 g (84%) of light yellow AB3-G1. 1H NMR (ppm, CDCl3) δ =0.51–0.80 (m, CH2), 1.43–1.92 (m, CH2), 3.60–4.01 (m, OCH2), 4.29 (s, OCH2), 4.91–5.41 (m, =CH2), 5.72–6.12 (m, =CH).
3.5 Preparation of AB3-9Cl
The same procedure as that for the preparation of AB3-G1-3Cl was used in the reaction of 4.00 g (69.47 mmol) of AB3-G1, 10.66 g (69.47 mmol) of HSiCl3 and 0.10 g (1.30 mmol) of Pt/C. 1H NMR (ppm, CDCl3) δ = 0.80–1.09 (m, CH2), 1.32–1.62 (m, CH2), 1.62–2.10 (m, CH), 3.34–3.63 (m, OCH2), 3.63–4.12 (m, OCH2). 13C NMR (ppm, CDCl3) δ = 10.96–29.52, 44.91 (CH2), 63.34–66.51, 69.89–70.64 (OCH2).
3.6 Preparation of the second generation (AB3-G2)
The same procedure as that for the preparation of AB3-G1 was used in the reaction of 0.73 g (3.65 mmol) of AB3-G2-9Cl, 2.50 g (43.06 mmol) of allylalcohol and 2.00 g (17.47 mmol) of TMEDA. The product was purified by chromatography on a silica gel with chloroform with a mixed eluent (CHCl3: THF = 9:1). Yield: 1.12 g of a colorless gel. 1H NMR (ppm, CDCl3): δ = 1H NMR (ppm, CDCl3) δ = 0.50–1.12 (m, CH2), 1.20–1.40 (m, CH2), 1.64 (s, CH2), 3.12–4.00 (m, OCH2), 4.29 (s, OCH2), 4.98–5.20 (m, =CH2), 5.80–6.12 (m, =CH). 13C NMR (ppm, CDCl3) δ = 6.11–29.43, 44.72–44.80 (CH2), 62.50–65.68, 69.73–70.79 (OCH2), 114.52–114.75 (=CH2), 136.03–136.36 (=CH).
Acknowledgments
This study was supported by a grant from Basic Science Institute of Dong-A University (2003).


