1 Introduction
Extraction of natural products with supercritical carbon dioxide (SCCO2) has gained increasing attention from the food and perfumery industries to obtain flavour and fragrance ingredients as well as the pharmaceutical industry, namely because the dissolving power of supercritical fluid can be adjusted by regulating its pressure and temperature [1–4]. Also, by using SCCO2 instead of steam distillation or extraction with organic solvents (hexane, chloroform) eliminates the problem of toxic residual solvent in the products, and permits the use lower temperatures leading to lesser deterioration of the thermally labile components in the extract. Likewise, extraction with SCCO2 retains the organoleptic characteristics of the starting spice materials [5]. In addition, the modifier – a small amount of polar organic solvent added to the supercritical CO2 –, will increase overall extractability of target analytes [6].
The leaves of plants such as sage, thyme and oregano have been added to meat, fish and food products for seasoning. It is known that in addition to improving flavour, certain spices and essential oils prolong the storage life of foods. The antimicrobial activities of various spices [7,8] and of essential oils obtained by hydrodistillation methods [9] have been reported.
In the present study, SCCO2 was used to extract the volatile aroma components from four herbs: sage, basil, oregano and lovage. Since different targeted compounds have different diffusion rates inside the plant matrix [10], and the solubility of every substance in the supercritical fluid depends on extraction pressure, temperature and modifier, data about yield provide a diagnostic tool for further optimisation of the extraction process. We also report here the antimicrobial properties of supercritical extracts.
2 Materials and methods
2.1 Plant material and chemicals
The herbs: sage (Salvia officinalis), basil (Ocimum basilicum), oregano (Origanum vulgare), lovage (Levisticum officinale) were grown in Estonia and collected in July 2002. The raw material was dried at room temperature (approximately 22 °C) and protected from direct light.
The carbon dioxide (Eesti AGA, Estonia) used in the experiments was 99.5% (w/w) pure. The ethanol as modifier used was spectroscopic grade (Liviko, Estonia) and added according to weight of the sample (from 2.5 to 7%).
2.2 Extraction of herbs
Supercritical fluid extractions from dried leaves or/and flowers of plants were performed on Sample Preparation Accessory apparatus (Milton Roy SPA, USA). Extractions were carried out at 45 °C, at pressures from 17.2 to 25.5 MPa. The flow rate of supercritical CO2 was 1 ml min–1 and extraction time for all experiments was 60 min. The extract was collected in chloroform. Extraction time (1 h) was chosen on the basis of previous studies [11]. There was estimated, that at this pressure range used the yield was ~85% of total yield able to achieve during 4-h extraction.
For comparison, a conventional steam distillation using a Clevenger apparatus for 3 h was utilized for the isolation of the essential oils. After steam distillation, the essential oil was isolated and kept refrigerated.
2.3 Gas chromatography (GC)
Chemical composition of extracts was determination by gas-liquid chromatography method using a fused silica capillary column with a bonded stationary phase NB 30 (HNU Nordion, Finland), and flame ionisation detector. The identification was performed by comparing their Kovats relative retention indexes (RI) with those of authentic compounds and from database collected from literature.
2.4 Assay for antimicrobial activity
The microorganisms: Bacillus mesentericus, Staphylococcus albus, Escherichia coli, Aspergillus flavus and Penicillium sp. were used in this study. Laboratory strains, kept on the solid agar at 4 °C, were used. Antimicrobial activity was tested by the filter paper disc diffusion method [12]. A 5.5-mm sterile paper disc was impregnated with a test material and set on agar using a micropipette. Plates were then inverted and incubated for bacteria – at 30 °C for 48 h, for moulds – at 25 °C for 96 h. Following incubation, the zones of inhibition were measured (in mm s).
3 Results and discussion
3.1 Effect of pressure on the extract yield
Preferable extraction conditions were 17.2 MPa for sage and lovage, and 25.5 MPa for basil and oregano (Table 1). The total maximum yield of extract for 1-h extraction time for sage was 13.2 mg g–1, for lovage 5.2 mg g–1, for basil 4.4 mg g–1, and for oregano 4.1 mg g–1. A decrease in yields of the extracts for sage and lovage at increased pressures is hard to explain. It was found in all experiments made at the same conditions but in different time. The similar results, where system behaviour cannot be solely attributed to the effects of either temperature or pressure, have been reported in the previous study on SCCO2 extraction of herbs [13], where it was found to be specific for sage only. Later from the literature, the same kind of unusual behaviour was found in case of extraction of stevia leaves [14]. Possibly, here could be some structure changes or textural transformations in the plant matrix at the higher extraction pressure, which would hinder the diffusion, and practically it means that longer extraction times are needed. Another explanation could be in some cumulative effect of extract components solubility changes, but this needs further study on solubility data in supercritical CO2 of the main components in lovage extract, and estimation of phase equilibrium data is necessary for the system.
Total yield (mg g–1) and relative peak area (%) from GC analysis of main constituents of plant extracts at different pressures (17.2 and 25.5 MPa, at 45 °C, extraction time 60 min, each experiment repeated three times)
| Sage | Basil | Oregano | Lovage | ||||
| 17.2 MPa | 25.5 MPa | 17.2 MPa | 25.5 MPa | 17.2 MPa | 25.5 MPa | 17.2 MPa | 25.5 MPa |
| Total yield (mg g–1) | |||||||
| 13.2 ± 1.0 | 7.6 ± 0.8 | 3.8 ± 0.4 | 4.4 ± 0.4 | 3.2 ± 0.4 | 4.1 ± 0.4 | 5.2 ± 0.4 | 4.8 ± 0.5 |
| Main constituents (area%) | |||||||
| Camphene | Linalol | Thymol | α-Terpinyl acetate | ||||
| 6.1 | 5.1 | 52.0 | 42.5 | 7.2 | 11.2 | 64.1 | 41.3 |
| β-Pinene | Methyl-eugenol | Carvacrol | β-Phellandrene | ||||
| 9.5 | 0.1 | 22,5 | 15.1 | 8.0 | 10.9 | 12.4 | 0.1 |
| 1,8-Cineole | (E)α-Bergamotene | Linalol | Myrcene | ||||
| 9.7 | 12.9 | 6.5 | 4.6 | 3.7 | 5.6 | 2.3 | 0.1 |
| α-Thujone | 1,8-Cineole | Thymol acetate | Phthalide isomer | ||||
| 27.1 | 18.6 | 4.5 | 10.3 | 0.1 | 12.4 | 7.6 | 5.9 |
| Camphor | |||||||
| 15.6 | 18.9 | ||||||
| Other minor constituents (area%) | |||||||
| 32.0 | 44.4 | 14.5 | 27.5 | 81.0 | 59.9 | 13.6 | 52.6 |
The important conclusion is that one must be very careful with extrapolating data from other experiments, and must check the yield dependence from extraction time at higher pressures for certain plants.
3.2 Effect of ethanol as modifier
Although carbon dioxide is a relatively good solvent for the extractions, it has some limitations for the extraction of polar substances from plant matrix. Ethanol was used as modifier in this study, because it is environmentally benign and relatively safe to human health. The amount of ethanol added initially composed 2.5, 5 and 7.5% of the total sample weight.
Fig. 1 shows that an increased ethanol amount increases the extraction yield from the plants, however, for lovage again, the yield decreased with increase the amount of the modifier. It points that processes during the extraction at higher pressures and with solvent mixtures are complicated, and the interaction of the co-solvent with the cellulosic structure or solid matrix of the plant cannot be neglected [14], but more likely, the components in lovage extract cumulatively might not be as soluble in the mixture of SCCO2 and ethanol at higher pressures and modifier concentrations.
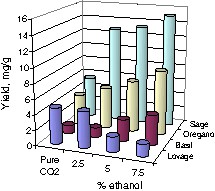
Influence of modifier addition on the total yield of extract (45 °C, 25.5 MPa) for different herbs.
3.3 Comparative analysis of plant components at different extraction pressures
The chemical composition of the SCCO2 extracts from these plants was determined by GC. The total yield of extracts and relative content of main compounds are presented in Table 1. It confirms that every plant has its own unique extract composition.
GC analyses revealed that the main constituent of the extract from sage at 17.2 MPa is α-thujone, but its relative content decreases at 25.5 MPa, as well as its β-pinene content. Thymol and carvacrol are the main components in the oregano extract. Lovage extract is characterized by the relatively high content of α-terpinyl acetate and β-phellandrene. Linalol is the main constituent in the basil extract. Despite the higher total yield of basil at 25.5 MPa compared to pressure 17.2 MPa, the percentage of the major components (except 1.8-cineole), is less. It means that at higher pressures the additional components are extracted and content of extract is changing substantially. Some of them are difficult to analyse by GC methods normally used for essential oils, and additional chromatographic methods might be instructive.
In conclusion, the compositions of these extracts depends on the extraction conditions, hence SCCO2 extracts cannot be identified with the essential oils from the plants. Some researchers have recommended temperatures between 40 and 50 °C and extraction pressures lower than 100 bar (9.9 MPa) for obtaining extracts that are similar to essential oils from plant materials [15]. Our results confirm that for each aromatic plant, specific extraction conditions are needed to obtain, especially for an extract similar to that essential oil by conventional methods, and higher extraction pressures and addition of modifier increase the yield of the extract with changed proportion of components.
3.4 Antimicrobial activity of supercritical extract
There is not very much data about the antimicrobial activity of supercritical extracts from herbs, and those that exist concern extracts obtained using traditional methods, such as hydrodistillation [10]. In this study, the antimicrobial activity was tested with extracts from sage; the studies with extracts from other plants are ongoing. Here sage showed the highest inhibitory effect against gram-positive bacteria and a weakly inhibitory effect against moulds. Sage showed no effect against gram-negative E. coli.
For sage it was possible to compare the activity of SFE extract and that from the essential oil obtained using steam distillation/extraction method (Fig. 2a and b). One explanation for this different behaviour is the difference in composition of extracts, as shown for sage in Table 2.
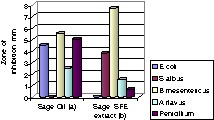
Comparison diagram of antimicrobial activity of sage essential oil and sage SCCO2 extracts (17,2 MPa, 45 °C) for five different microorganisms.
Comparison table of chemical composition of sage essential oil and SFE extract (%)
| Component | Essential oil | SFE extract (17.2 MPa, 45 °C) |
| α-Pinene | 4.9 | 5.3 |
| Camphene | 5.0 | 6.1 |
| β-Pinene | 3.4 | 9.5 |
| 1,8-Cineole | 12.1 | 9.7 |
| α-Thujone+linalool | 21.2 | 27.1 |
| β-Thujone | 4.4 | 4.4 |
| Camphor | 23.6 | 15.6 |
| Borneol | 5.6 | 2.1 |
| (E)-β-Caryophyllene | 2.7 | 2.2 |
| α-Humulene | 5.2 | 4.9 |
| Viriflorol | 3.0 | 1.6 |
| Other | 8.5 | 7.9 |
| Total | 99.6 | 96.4 |
There is more α- and β-pinene, and also α-thujone in the sage SCCO2 extract, than in the essential oil. It may be supposed that these compounds have higher antimicrobial activity against gram-positive bacteria, or that there is some synergy between the extract components resulting in the observed antimicrobial activity.
4 Conclusions
Extraction conditions: CO2 pressure and amount of modifier should be optimised to obtain extracts with attractive properties. Extraction pressure is a very significant parameter; however, an increase of the pressure and addition of a modifier does not always has a positive effect on the yield of extracts (over the same extraction time). Specific studies on the extract of sage showed a difference between the SCCO2 extract and the native essential oils concerning antimicrobial activity. Further research is needed in order to obtain more reliable results on determination of antimicrobial activity of supercritical extracts.


