1 Introduction
Extraction and purification of U and Pu in spent nuclear fuel reprocessing (SNF) is a key chemical process in the nuclear industry. The most widely used commercial process to achieve this objective is the Plutonium Uranium Reduction EXtraction process (PUREX), which involves the dissolution of SNF in nitric acid followed by solvent extraction of U(VI) and Pu(IV) using tri-n-butyl phosphate (TBP) as extractant [1,2]. The PUREX process, though highly efficient, has the inherited drawbacks of liquid–liquid extraction, including generation of large volumes of aqueous and organic high-level radioactive wastes. So a search for new alternative techniques for reprocessing of SNF, which will allow minimizing waste generation, is a major task, challenging the future of the nuclear industry.
The possibility of using (SF-CO2) as a solvent for reprocessing of SNF was suggested recently [3]. Of particular interest is the idea of direct dissolution of SNF in SF-CO2 using a suitable complexing agent that should form a SF-CO2 soluble complex with the main components of SNF, basically with uranium dioxide. SF-CO2 is considered as a green solvent because it is non-toxic and environmentally benign. It is also cheap, readily available in relatively pure form, and has moderate critical constants (Tc = 31.3 °C, Pc = 72.8 atm, and ρc = 0.47 g cm–3) [4]. A major advantage of using supercritical CO2 for reprocessing of SNF is the elimination of the use of acid and organic solvent required in the conventional PUREX process.
The hexavalent uranyl ion UO22+ is known to form SF-CO2 soluble complexes with a number of complexing agents, including TBP and β-diketones [5]. It has been demonstrated that UO3 can be dissolved in SF-CO2 using thenoyltrifluoroacetone (HTTA) and TBP [6,7]. Recent studies have also demonstrated the possibility of lanthanide extraction from their oxides using SF-CO2 containing the TBP–HNO3 complex [8]. However, the extraction efficiency of all elements involved did not exceed 40%.
We have reported earlier [9] about the quantitative dissolution of milligram amounts of UO3 in SF-CO2 containing HTTA and TBP, which was performed using ultrasonication. Also, it was reported that uranium can be quantitatively extracted and efficiently separated from Pu, Np and Th in the process of SFE of the mechanical mixture of their dioxides using SF-CO2 modified with TBP–HNO3 complex [10].
The study of dissolution of solid solutions of actinide dioxides in UO2 using the system SF-CO2–TBP–HNO3 is the purpose of this work. Preliminary results obtained are presented below in comparison with previous results.
2 Experimental
Commercial oxides were used in the above-mentioned research about the dissolution of individual actinide oxides in SF-CO2 containing the TBP–HNO3 complex [9,10]. In the present work, we used the synthesized samples of solid solutions of actinide dioxides and Eu2O3 in UO2. Their preparation was carried out in accordance with the scheme presented in Fig. 1. According to [1,11] Np and Pu dioxides are formed in the process of air calcination of many Np and Pu compounds, such as hydroxide, acetate, nitrate and oxalate. Though Np and Pu may have any oxidation state in these compounds, the product of their calcinations is always NpO2 and PuO2. Since UO2 was the basis of all samples of the solid solutions of actinide dioxides prepared, it should be obtained in the form of pure UO2. For this purpose, drying, decomposition, and calcinations of mixed actinide oxalates up to the solid solution of their oxides were conducted under argon–hydrogen atmosphere. These operations were performed in glass-graphite crucibles, increasing the temperature from 20 to 850 °C for 5 h. Further calcination was continued for 8 h at 850 °C. In the case of U–Pu–Eu, about a half of the mixed oxide sample prepared was additionally calcined at 1200 °C for 8 h in order to simulate samples that may be obtained from real SNF, since the temperature in the fuel elements in the nuclear reactor can reach 1200 °C. A schematic diagram of the experimental set-up for SFE of actinide oxides, the preparation technique of the TBP–HNO3 complex and experimental procedures were described earlier [9,10]. Types and composition of the solid solutions of the corresponding actinides and Eu are presented in Table 1.
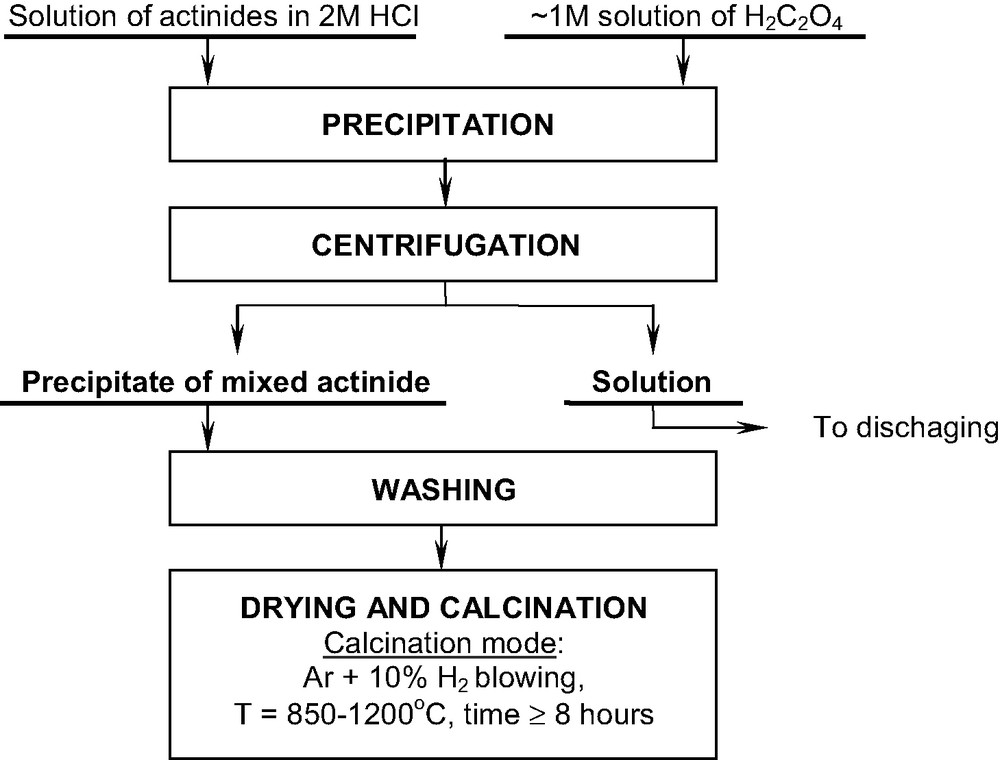
Preparation of the solid solutions of mixed actinide and Eu oxides.
Samples of the solid solutions of actinide and Eu oxides
| Composition of the solid solutions | Weight % |
| AmO2 | 10.7 |
| UO2 | 89.3 |
| NpO2 | 5.0 |
| UO2 | 95.0 |
| PuO2 | 5.0 |
| UO2 | 95.0 |
| PuO2 | 25.0 |
| Eu2O3 | 5.0 |
| AmO2 | 0.05 |
| UO2 | 69.95 |
3 Result and discussion
The results of X-ray structure analysis (Table 2) of the prepared U–Pu oxides carried out by the method of the X-ray powder diffraction pattern have shown that, in all cases, UO2 was the matrix of the mixed oxides. Unit cell parameters for the samples #1, #2, and #3 are equal to 5.4703 Å, 5.4232 Å, and 5.4421 Å, respectively. Reference data for UO2 are taken from the database PDF-2 (JCPDS-ICDD, 1999). According to it, a(Å) = 5.4704 – 0.0935 x, for UO2+X at 25 °C. An increase of Pu content in sample #2 in comparison with #1 results in a considerable decrease in the unit-cell parameter of UO2. At the same time, additional calcination of the sample #3 at 1200 °C results in its increase. Potentially, these changes in the unit-cell parameter value may influence the processes of dissolution of actinide dioxides in the course of SFE.
Interplanar spacing d(Å) and relative intensities (Int) for solid solutions of U–Pu oxides prepared and reference data for UO2
| Reference data for UO2 | Measured data for the samples prepared | ||||||
| U–Pu(5%) (Sample 1) | U–Pu(25%)–Eu (Sample 2) | U–Pu(25%)–Eu (Sample 3) | |||||
| Tcal = 850 °C | Tcal = 850 °C | Tcal = 1200 °C | |||||
| d (Å) | Int. (%) | d (Å) | Int. (%) | d (Å) | Int. (%) | d (Å) | Int. (%) |
| 3.156 | 100.0 | 3.154 | 100.0 | 3.135 | 100.0 | 3.147 | 100.0 |
| 2.733 | 34.7 | 2.729 | 44.4 | 2.716 | 50.0 | 2.725 | 50.0 |
| 1.932 | 40.9 | 1.934 | 56.4 | 1.920 | 70.0 | 1.927 | 70.0 |
| 1.648 | 35.0 | 1.649 | 63.6 | 1.636 | 70.0 | 1.642 | 70.0 |
| 1.578 | 7.6 | 1.579 | 18.4 | 1.570 | 10.0 | 1.572 | 20.0 |
| 1.366 | 5.0 | 1.368 | 14.2 | 1.359 | 10.0 | 1.361 | 10.0 |
| 1.254 | 11.5 | 1.255 | 29.9 | 1.245 | 30.0 | 1.249 | 30.0 |
| 1.222 | 8.5 | 1.224 | 23.4 | 1.214 | 20.0 | 1.217 | 20.0 |
We present in Tables 3 and 4 the results obtained earlier [9,10] of SFE of actinides from their individual and mixed oxides using SF-CO2 modified with the TBP–HNO3 complex. The new results on SFE of U, Np, Pu, Am and Eu from the solid solutions of their oxide are presented in Table 5. As seen from the data presented (Tables 3 and 4), uranium can be quantitatively extracted from all the oxides investigated. It was shown by spectrophotometry that uranium is present in the oxidation state +6 only in the trap solution (Fig. 2). Therefore, the interaction of TBP–HNO3 complex with UO2 results in the oxidation of the tetravalent uranium to the hexavalent one. This leads to the formation of highly soluble UO2(NO3)2·2 TBP complex in the SF-CO2 phase. Oxidation of Np and Pu up to the oxidation state +6 under the conditions investigated does not occur. So, the extraction efficiencies of Pu, Np, and Th from both individual oxides and their mixtures with uranium dioxide are extremely poor.
SFE of actinides from their individual oxides using SF-CO2 with the TBP–HNO3 complex. T = 65 °C, P = 250 atm
| Oxide | Actinide added (mg) | Molar ratio An:complex | Extraction efficiency (%) |
| UO2 | 61,5 | 1:50 | > 99 |
| 334.4 | 1:10 | 90 ± 5 | |
| 367.9 a | 1:10 | 65 ± 3 | |
| UO3 | 175.1 | 1:20 | 92 ± 5 |
| U3O8 | 177.3 | 1:20 | 85 ± 3 |
| PuO2 | 8.1 | 1:250 | < 0.1 |
| 50.1 | 1:50 | < 0.1 | |
| NpO2 | 5.6 | 1:250 | < 0.1 |
| 55.0 | 1:50 | < 0.1 | |
| ThO2 | 52.9 | 1:50 | < 0.1 |
a Non-milled uranium fuel pellet.
Separation of U from Pu, Np, and Th using SF-CO2 with the TBP–HNO3 complex T = 65 °C, P = 250 atm, molar ratio An:complex ~ 1:20
| Mixture of oxides | Actinide added (mg) | Extraction efficiency (%) | Separation factor (SU/An) |
| UO2 | 150.5 | 87 ± 4 | ~1200 |
| PuO2 | 37.4 | < 0.1 | |
| UO2 | 120.6 | 91 ± 5 | ~1400 |
| NpO2 | 11.5 | < 0.1 | |
| UO2 | 133.5 | 89 ± 4 | ~1100 |
| ThO2 | 58.5 | < 0.1 |
Dissolution of solid solutions of mixed radionuclide oxides in SF-CO2 with the TBP–HNO3 complex Vcomplex = 3 ml (0.01 mol), T = 65 °C, P = 200 atm
| Calcination temperature (°C) | Solid solution of mixed oxides | Wt % | Actinide content in oxide (mg) | Extraction efficiency (%) |
| 850 | AmO2 | 10.7 | 0.55 | 92.0 |
| UO2 | 89.3 | 4.56 | 98.2 | |
| 850 | NpO2 | 5.0 | 0.25 | 84.0 |
| UO2 | 95.0 | 4.70 | 93.6 | |
| 850 | PuO2 | 5.0 | 0,67 | 89.0 |
| UO2 | 95.0 | 14,60 | 93,3 | |
| 850 | PuO2 | 25.0 | 2.15 | 86.1 |
| UO2 | 69.95 | 6.14 | 94.4 | |
| Eu2O3 | 5.0 | 0.43 | 93.6 | |
| AmO2 | 0.05 | 0.0037 | 86.0 | |
| PuO2 | 25.0 | 3.91 | 89.6 | |
| 1200 | UO2 | 69.95 | 11.17 | 93.1 |
| Eu2O3 | 5.0 | 0.78 | 91.0 | |
| AmO2 | 0.05 | 0.0066 | 88.0 |
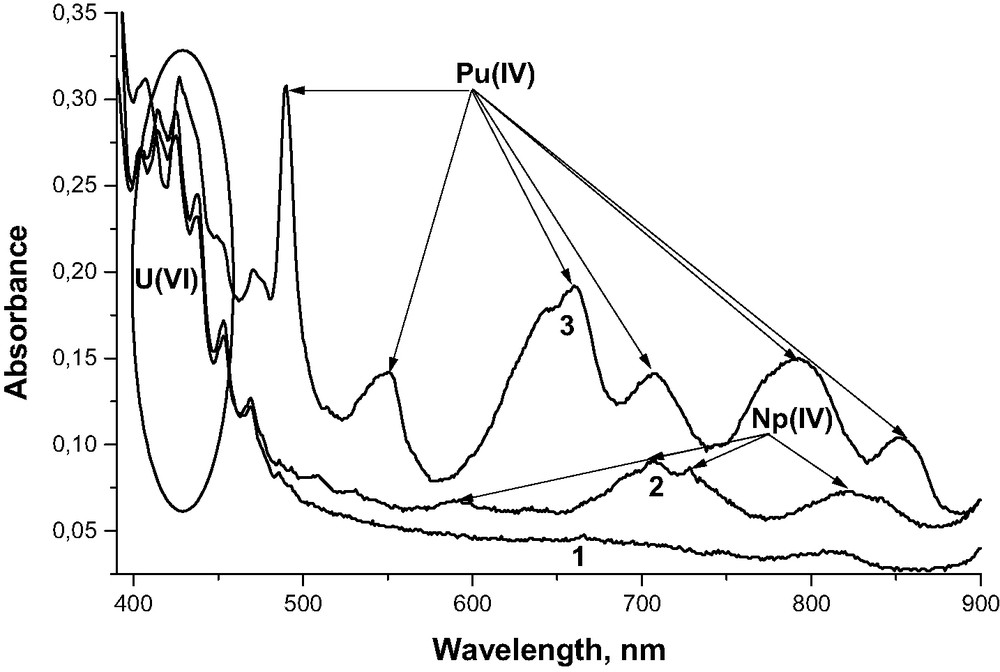
Absorption spectra of the trap solutions obtained from SFE of UO2 (1) and of solid solutions of mixed U–Np (2) and U-Pu (3) dioxides.
At the same time, it can be seen from the data presented in Table 5 that during SFE of the solid solution of U–Np, U–Am, U–Pu, U–Pu–Eu oxides, practically quantitative extraction of Np, Pu, Am and Eu occurs. It turned out that the Np and Pu passing into the trap solution exists in the oxidation state +4 (see Fig. 2). Other oxidation states of Np and Pu in absorption spectrum of this solution are not seen on the spectra. It means that during the contact of the TBP–HNO3 complex with the solid phase of U-Pu or U-Np oxides U(IV) easily oxidizes to U(VI), resulting in breaking the structure of the actinide solid solutions and in initiating the formation of the SF-CO2-soluble complexes of Pu(IV) and Np(IV) with the TBP–HNO3 complex. The results obtained show that trivalent europium and americium pass quantitatively into the trap solution as well. Increase in the Pu content in the matrix of UO2 from 5 to 25% has practically no effect on the relative yield of U and Pu on SFE for solid solution or their mixed dioxides under the conditions investigated. An increase of the calcination temperature of the mixed U–Pu–Eu oxide from 850 to 1200 °C has no influence on the relative yield of radionuclides involved.
4 Conclusion
Uranium can be quantitatively extracted from UO2, UO3 and U3O8 with SF-CO2 containing the TBP–HNO3 complex. On dissolution of UO2 in the system, the oxidation of U(IV) to U(VI) proceeds with the formation of the SF-CO2-soluble complex UO2(NO3)2·2 TBP. Uranium can be efficiently separated from Pu, Np and Th in the course of SFE from mechanical mixtures of their oxides. Contrary to the mechanical mixtures of actinide oxides, solid solutions of mixed actinide oxides are quantitatively dissolved in SF-CO2 containing the TBP–HNO3 complex. Pu and Np trapped during SFE of solid solutions of mixed oxides turn out in the trap solution in the oxidation state +4, whereas U is in the oxidation state +6. An increase in the Pu content in the matrix of UO2 from 5 to 25% has practically no effect on the relative yields of U and Pu on the SFE of the solid solution of their mixed dioxide under the conditions investigated. An increase in the calcination temperature of the mixed U–Pu–Eu oxide from 850 to 1200 °C has no influence on the relative yield of radionuclides involved during SFE as well. Application of SFE technique may be very promising for SNF reprocessing
Acknowledgements
This work was supported by Russian Foundation for Basic Research, grants # 99-03-32819 and 00-15-97391.


