1 Introduction
Soil waterlogging initiated by both natural (floods, snowmelt) and anthropogenic factors (melioration, waterworks) strongly affects all components of the soil as well as soil-forming and migration processes [1–3]. Study of the migration behaviour of macro- and microelements in waterlogged soils is important to gain a better insight into the mechanisms of natural attenuation in contaminated areas.
Presently, available data on the potential mobility of technogenic radionuclides in soils and sediments are obtained mostly by the sequential extraction methods [4,5].
We demonstrated previously that synthetic mixed Fe, Al, Ca fulvate can be used as a model for studying the migration behaviour of 241Am and 152Eu in soils over wide condition ranges [6]. Our model experiments showed that the mobility of heavy metal nuclides in soils is controlled to a considerable extent by the stability of the coordination-polymeric structure of organomineral complexes formed by humus acids with the major soil metals (Fe, Al, Ca, Mg) [7,8]. It should be pointed out that the presence of calcium considerably decreases the solubility of these complexes, which is consistent with the concept of the role of calcium in the formation of waterproof soil aggregates [9].
The behaviour of metals in waterlogged soils is strongly affected by the development of reducing processes through anaerobic metabolism of heterotrophic microorganisms [1–3,10,11]. Under anoxic conditions, mobile Fe(II) complexes with humus acids are formed, initiating partial solubilisation of the organomineral films (hereinafter called films) composed of iron-containing amorphous hydroxides and humates and fulvates of the major soil metals (Fe, Al, Ca) and fixed onto the surface of clay particles [3,11–14]. This process is known as gleysation. Bicarbonate and low-molecular-weight organic acids also contribute significantly to mobilization of organomineral complexes. As known, the organomineral films are structural elements of the soil that fix most actively toxic metals and radionuclides [12]. It should be expected that solubilisation of the films is accompanied by joint leaching of iron and other elements and radionuclides, among them those adsorbed as a result of technogenic contamination. This effect is not practically studied yet.
Therefore, in this work we studied joint leaching of iron and other elements, from radioactively contaminated waterlogged soils with the aim of searching for new effective soil remediation technologies based on accelerated natural attenuation.
2 Experimental
All experiments were performed with the soil collected from the floodplain of the Yenisei River downstream of the Krasnoyarsk Mining and Chemical Combine. The contents of environmentally significant radionuclides were as follows (Bq kg-1): 239,240Pu 2.14, 60Co 193, 137Cs 3790, and 152Eu 187. Other characteristics of the sample: contents of elements (wt%): Na 3.0, K 1.5, Mg 1.2, Ca 1.1, Al 4.6, and Fe 2.8; organic carbon 1.1 wt%; humic to fulvic acid ratio 1.57; pH 7.2; CEC 21.0 mg equiv/100 g.
Leaching of elements from the soil was studied with an experimental set-up allowing a permanent saturation level of the soil moisture in the stagnant-drainage flow mode (Fig. 1).
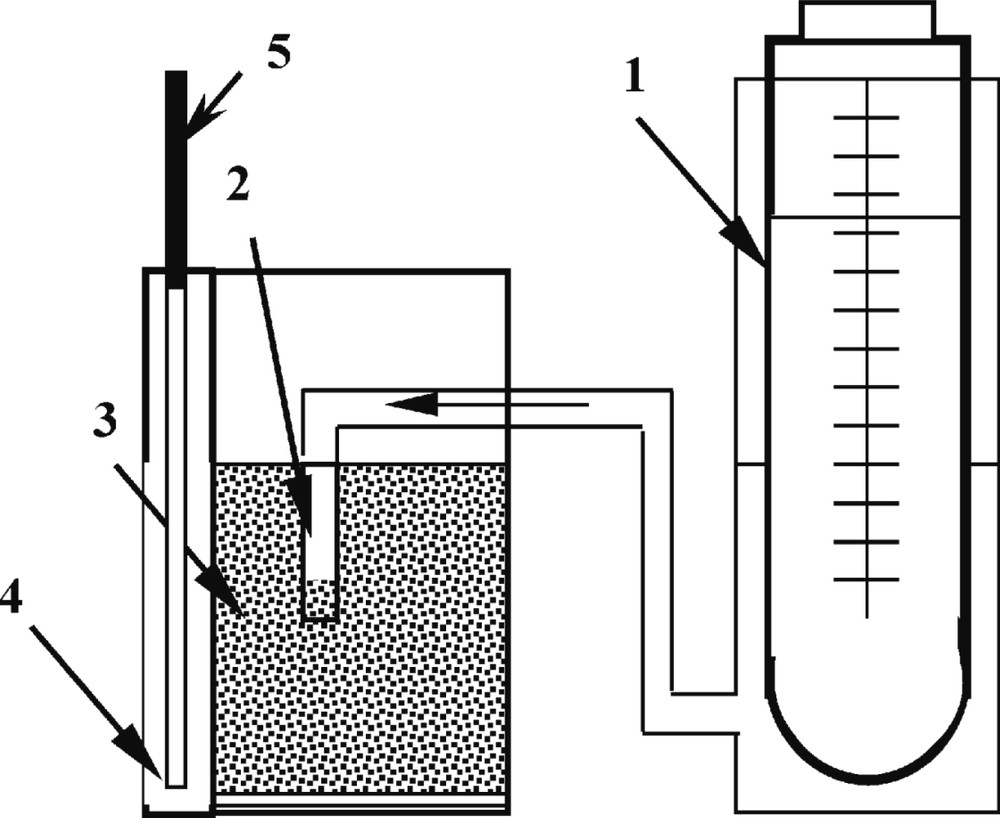
Experimental set-up for studying the mobility of elements in waterlogged soils: (1) manostat, (2) moistener, (3) soil sample, (4) receiving vessel, and (5) filter-concentrator.
The test soil sample (100 g) is placed into a cell with a filter bottom (chemically resistant dense cloth). The head gradient is kept constant using a manostat filled with a liquid phase of interest. The liquid phase drains from the sample into a receiving vessel, and then moves up by capillarity along a high-density paper filter (blue tape) vertically arranged in the receiving vessel. The driving force of the process is evaporation of water from the top of the receiving filter. As a result, all the species significant on the migration viewpoint formed in the system, including soluble forms and pseudocolloids, are concentrated at the top of the receiving filter. In the steady mode, the liquid phase flow is equal to the evaporation rate of water from the top of the filter. This set-up proved to be quite convenient for conducting long-time experiments, to study the effect of biochemical factors on the speciation and mobility of elements in soils. It furnishes information on the integral leaching of mobile components with permanent control of pH and Eh in the system.
The pH and Eh were measured, respectively, with glass and Pt combined electrodes on an Orion Research Model 611 pH/millivoltmeter.
To estimate the leaching dynamics, the removable receiving filters were burned at 600 °C. The ash residue was digested with a mixture of concentrated nitric and hydrofluoric acids. Metals were determined by ICP-MS [(ICP-MS VG Plasma Quad (UK)]. The determination error at an element content of tens ppb was within 15%. 239,240Pu was determined by radiochemical analysis with 242Pu as a tracer. 60Co, 137Cs, and 152Eu were determined on a low-background gamma-ray spectrometric system.
3 Results and discussion
Data on the leaching of stable elements and radionuclides in a two-month experiment are given in Fig. 2. Under the experimental conditions (saturation moisture conditions; liquid phase flow 10–15 ml per day), Eh in the test sample decreased from the initial +385 mV to +140 mV by the end of the experiment (pH remained practically unchanged, being 7.1 ± 0.1). Decreasing Eh suggests developing reducing processes as a result of anaerobic conditions.
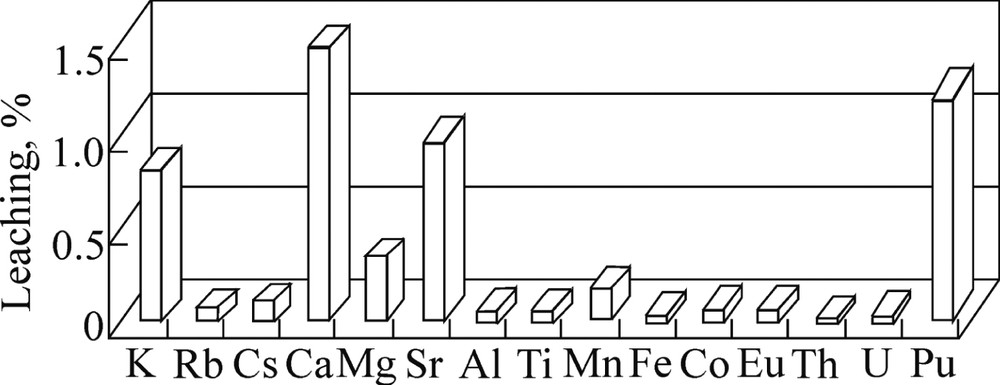
Leaching of elements from the test soil sample (100 g) with water (% from the amount extracted from a 100-g sample with 6 M HCl). Element contents in the acid extract (mg): K 61.4, Rb 0.9, Cs 1.7, Ca 223, Mg 500, Sr, 3.5, Al 1503, Ti 77.0, Mn 36.4, Fe 1348, Co 0.90, Eu 0.90, Th 0.12, and U 0.39. For plutonium, the leaching is given in % from its total content in the sample (0.21 Bq); the same for Fig. 3.
The leaching of elements varies over a wide range from 0.01% for Th to 1.5% for Ca. The leaching of 60Co, 137Cs, and 152Eu was below the detection limits (0.3, 0.016, and 0.5%, respectively). It should be pointed out that the observed percent of Pu leaching (1.3 ± 0.5%) is well comparable with that of Ca. This is important in view of the fact that plutonium is one of the most environmentally significant radionuclides.
As mentioned above, reduction of iron in the hydroxide and organomineral constituents of the films is one of the most important processes occurring in waterlogged soils. Since technogenic hydrolysable radionuclides are preferentially fixed just on the films, solubilisation of the films initiated by reductive mobilization of iron should be accompanied by remobilisation of radionuclides. Indeed, our results show the parallelism between the leaching of iron and other elements. This is illustrated in Fig. 3 with the examples of Pu and U.

Interrelation between the leaching of iron and that of Pu (a, b) and U (c, d). Liquid phase: water (a, c) and 0.4% (b, d) ascorbic acid.
Although data obtained with water as a liquid phase (Fig. 3a and c) quite clearly demonstrate parallelism in iron leaching, on the one hand, and Pu and U, on the other one, the absolute value of leaching is not high. Evidently, this is due to low enzyme activity in the tested clay sand soil poor in bio-available organics. Therefore, it was interesting to study leaching of elements from the same soil sample, but at lower redox potentials.
With this purpose, we used 0.4% aqueous ascorbic acid instead of water as a liquid phase. Selection of ascorbic acid was made taking into account that, as in anaerobic respiration, it reduces Fe(III) with the loss of two hydrogen atoms. Furthermore, ascorbic acid is non-toxic and can be involved in the nutrition chain of microorganisms. Finally, it was demonstrated in preliminary experiments that in the presence of 0.4% ascorbic acid, the redox potential in the system decreases to the values typical of highly developed anaerobic conditions in waterlogged soils [1,2].
To the end of a 60-day leaching experiment with 0.4% ascorbic acid, Eh decreased from the initial +385 mV value to –60 mV, and pH decreased to 4.7. These conditions promote reduction of Fe(III), whereas acidification and the presence of the ascorbate anions prevent formation of Fe(II) hydroxide. Data on leaching of elements with 0.4% ascorbic acid are shown in Fig. 4. As seen, changing water for ascorbic acid considerably intensifies the leaching of all the interested elements, the highest effects being observed for Fe, Mn, and Co, i.e., for the elements having capacity for reduction (Fig. 5).
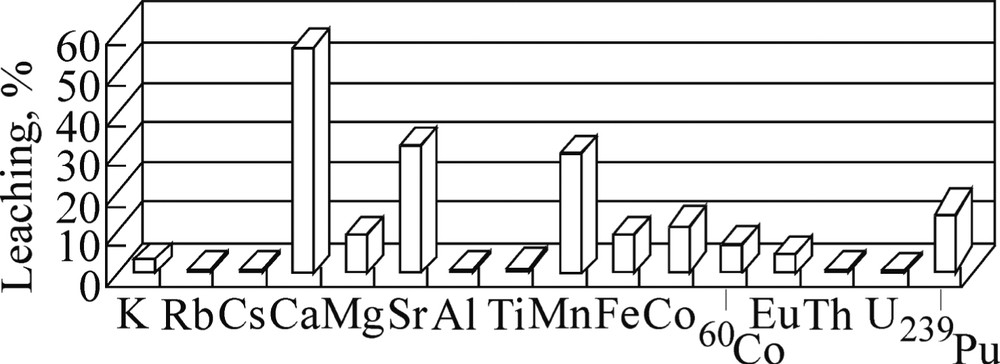
Leaching of elements from the test soil sample (100 g) with 0.4% ascorbic acid (% from the amount extracted from a 100-g sample with 6 M HCl).

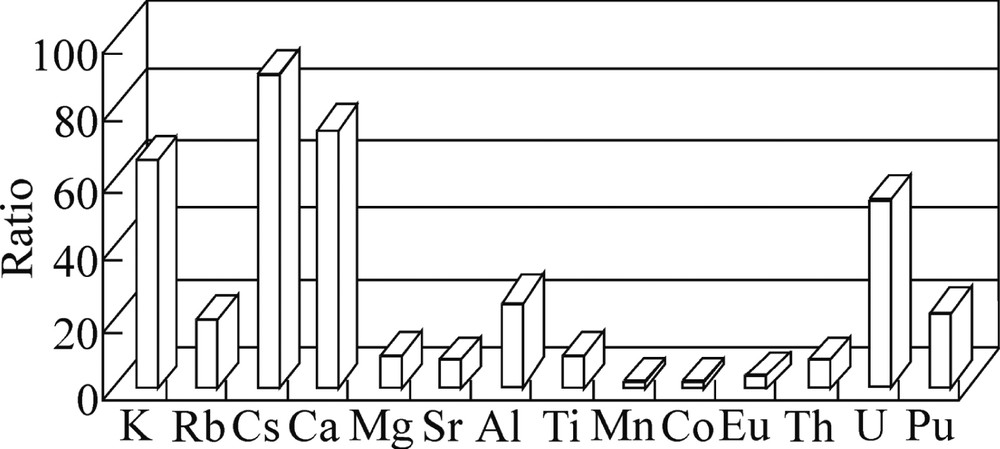
Ratio R between the leaching of elements with 0.4% ascorbic acid and water.
As in the experiments with water, we observed relatively high plutonium leaching (15.6%) with ascorbic acid. This result is consistent with data on plutonium partitioning in soils and bottom sediments, demonstrating that its mobile forms are associated essentially with the organomineral fraction, which represents a significant constituent of the films [15]. The parallelism in the behaviour of plutonium and iron is also reflected in their deposition in some illuvial horizons [16] and also in speciation in soils [17]. In contrast to other elements, leaching of 152Eu is not a monotonic function of the volume of the liquid phase passed. While the leaching of this nuclide in the first month was about 5%, in the second months, only less than 0.5% was leached.
In the experiments with ascorbic acid, we also observed direct correlations between the leaching of iron and Pu and U (Fig. 3b and d), counting in favour of the concept that, in waterlogged soils, gleysation promotes mobilization of both macro- and microelements.
Figs. 2 and 4 show high percent of leached calcium. Like iron, calcium takes an important part in soil-forming processes, controlling to a great extent the aggregate stability of the films. Leaching of these elements initiated by gleysation results in degradation of the waterproof structures and also in soil compaction. We demonstrated previously in model experiments with synthetic Fe, Al fulvates [6] that the aggregate stability of the model fulvate films increases in the presence of calcium. It was also shown [7,8] that decreasing the content of trivalent metals in the coordination-polymeric structure of the metal-fulvate gel phases below some critical level causes the loss in their aggregate stability, i.e., solubilisation of these phases. Our results suggest that, in the actual soils, solubilisation of the films, initiated by unbalanced removal of iron, proceeds by similar mechanisms, i.e., through degradation of the coordination-polymeric structure of organomineral complexes.
The conditions realized in our model system not only strongly influence the mobility of elements, but also initiate noticeable alteration of the group and fraction composition of the soil organic matter. According to our data, under the reducing conditions enhanced in the presence of ascorbic acid, all the fulvic acid fractions decrease. At the same time, the humic acid fraction HA2 associated with calcium increases, and the soil humus acquires a pronounced humate nature, which reflects the buffer capacity of the soil with respect to the transformation processes directed to its degradation. Evidently, the loss of fulvic acids by the soil is associated with the reduction of iron in the initial Ca,Fe,Al fulvates accompanied by their solubilisation. It is worth noting that the results obtained in the leaching experiments and data on the evolution of the composition of the organic matter are consistent with those obtained in studying natural gleysation [2].
Since we assumed that leaching of microelements proceeds by essentially the film solubilisation mechanism, it appeared interesting to compare the specific (related to unit quantity of leached iron) leaching of microelements with water Lw and aqueous ascorbic acid Lasc under gleysation conditions (Fig. 6).
Specific leaching ratio of elements with aqueous ascorbic acid and water.
It is clear that, if the leaching of iron and other elements proportionally increases on passing from water to aqueous ascorbic acid, the specific leaching ratio Lw/Lasc should be about unity. However, Fig. 6 shows that this ratio is considerably above unity for the most elements, i.e., in the presence of ascorbic acid, the specific leaching of elements decreases.
Keeping in mind that, with water as a liquid phase, pH in the system falls within the range of hydrolysis of Fe(II) [18], one should expect that the iron of ferric hydroxides can be reduced with formation of insoluble Fe(OH)2 (if no sufficient amount of complexants is available). On the contrary, transformation of poorly soluble complexes of Fe(III) with humus compounds into soluble complexes of Fe(II) does not require some additional ligands. Therefore, it should be expected that, under reducing conditions, in the range of hydrolysis of Fe(II), the iron fulvate complexes of the films more readily mobilize than the hydroxides. Evidently, in leaching with water, the most reactive iron species, i.e., organomineral and amorphous hydroxide constituents of the films, are primarily reduced. In the presence of ascorbic acid, more inert crystalline Fe oxide species can also be involved in reductive mobilization processes. Since these species are weaker sorbents for metals, in this case, increasing total leaching of iron is not accompanied by proportional increase in the leaching of other metals, including sorbed contaminants. To the least extent, this is related to Co and Mn, which is consistent with the known behaviour similarity between these elements and iron in the formation of the films and of crystallised oxide species.
Finally, the results obtained show that the aggregate stability of the organomineral films fixed on the surface of clay mineral particles does control to a considerable extent the mobility of elements in the soil. This is especially true for technogenic heavy radionuclides associated essentially with organomineral and amorphous hydroxide constituents of the films. Under reducing conditions, gleysation results in mobilization of both macro- and microelements through solubilisation of the films. Our thought is that this result is interesting in view of searching for new efficient site restoration processes on the basis of accelerated natural bioremediation.


