1 Introduction
The problem of radioactive wastes, accumulated as the result of production of nuclear energy and nuclear weapons in the past and their shortages nowadays is one of the important ecological problems to be solved. In the US, highly alkaline radioactive wastes (HARW) exist in aging tanks at Hanford, Savannah River, West Valley and Oak Ridge, and many tanks contain actinide elements in quantities of concern for separation, treatment and disposal in the USA [1,2] and in Russia [3–5]. Knowledge of actinide chemical properties under highly alkaline conditions is essential to understand and predict their solubility, sorption, phase distribution in tanks, to determine whether chemical separation are needed for waste treatment, and the design and optimization of rational separation process. The present work is devoted to the study of disproportionation of Pu(VI) and the properties of Am(III–VII) compounds formed in alkaline media.
2 Experimental
2.1 Interaction of Am(VI) with water
The reduction rate of 243Am(VI) in basic aqueous solution (~20% per hour) was found to be of the same order than for 241Am(VI). The values of the apparent rate constant (ka) of the first-order reaction in 3 M and 1 M NaOH are the same, i.e. ~(5.3 ± 0.3) × 10–4 s–1. In 0,1 M NaOH, ka is increased to 1.7 × 10–3 s–1. These data show that the reduction of Am(VI) by water molecules is the main contribution to the process and considerable contribution of Am alpha-radiolysis effects into the americium reduction in these conditions can be neglected.
2.2 Am(VII) species
Interaction of ~10–3 M 243Am(VI) solution in 1 M NaOH under mixing at 20 °C with sodium perxenate leads to a precipitate. The ozonization of this precipitate leads to the formation of Am green soluble species with specific optical spectrum (Fig. 1). It is much alike the spectra of Np(VII) and Pu(VII) in alkaline media [6], but shifted to the IR range. It is supposed that, in this experiment, an Am(VII) compound has been formed.
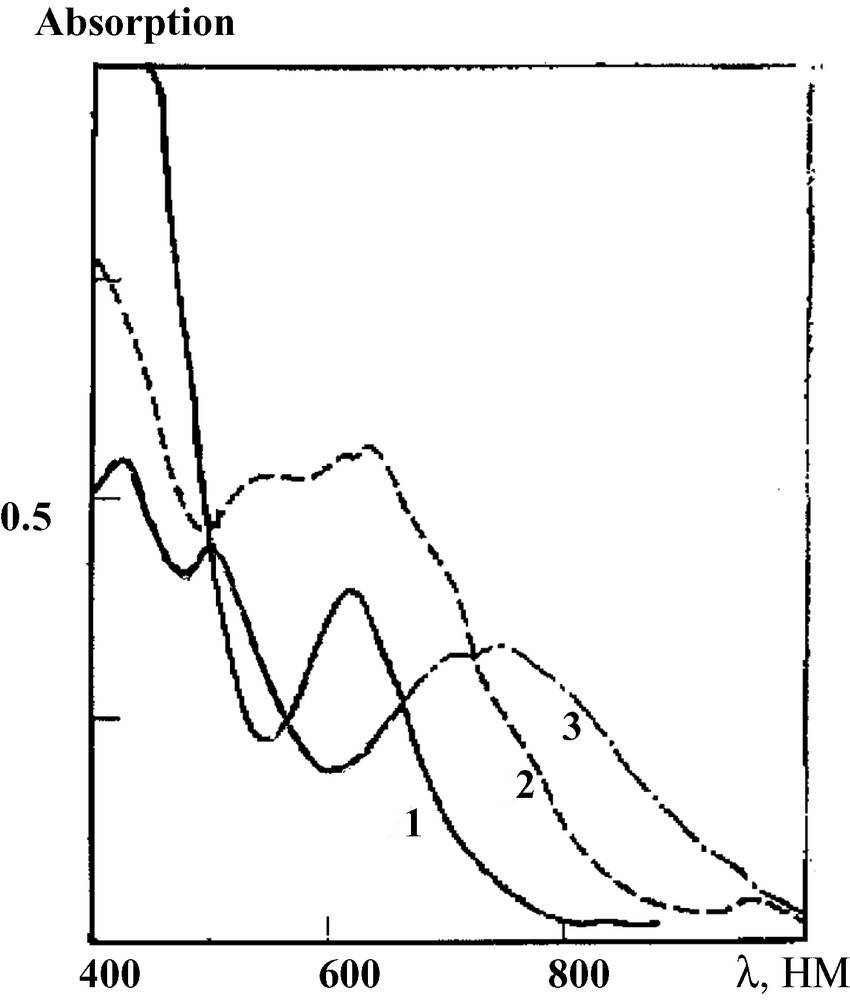
Absorption spectra of heptavalent Np [1], Pu [2] in 4 M NaOH [7], and ozonized Am:Xe complex obtained in our work [3].
2.3 Americium hydroxides interactions
The mixing of equivalent amounts of 243Am(III) and 243Am(V) hydroxides in 1 M NaOH at 50–70 °C, or in 7M NaOH at 25 °C during a few minutes led to the appearance of pure Am(IV) species via the reproportionation reaction Am(III) + Am(V) = 2 Am(IV). The absorption spectra of initial and final mixture are given in Figs. 2 and 3, respectively. The reaction between Am(VI) and Am(III) hydroxides in equivalent amounts brings the same result.
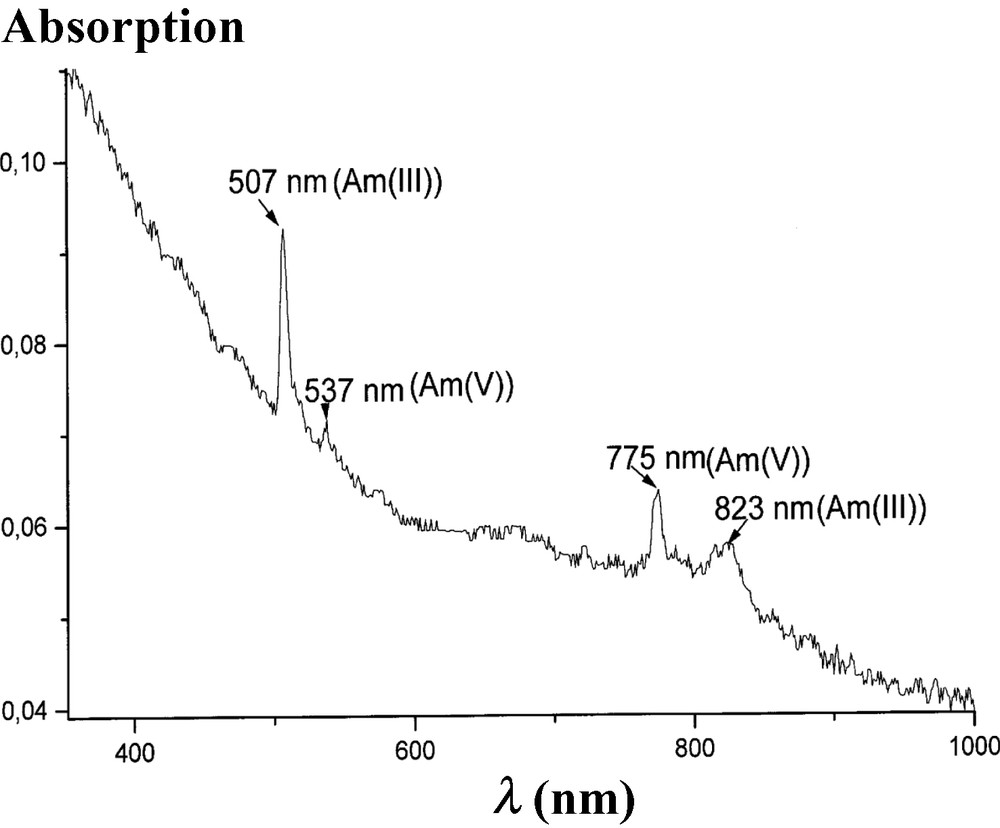
The absorption spectrum of solution in contact with mixture of Am(III) and Am(V) hydroxides in 1 M NaOH solutions at 25 °C.

Absorption spectrum of a solution in contact with a mixture of Am(III) and Am(V) hydroxides in 1 M NaOH at 70 °C or in 7 M NaOH at 20 °C.
2.4 Heterogeneous reversible Am redox reaction in alkaline media
In 0.006 M NaOH, an excess of K3Fe(CN)6 does not oxidize Am(III) hydroxide at room temperature. An increase of [NaOH] to 0.087 M leads to the reaction of 0.067M K3Fe(CN)6 and 1.5 mM of 243Am(OH)3 at 20 °C, with the formation of Am(OH)4. In 2 M NaOH solution, K3Fe(CN)6 (0.04M) oxidizes Am(OH)3 to Am(V). The redox reaction of Am(VI) and RuO42– ions is reversible in basic solutions. Based on the literature data on extinction coefficient of ruthenate and perruthenate ions and redox potential of RuO4–/RuO42– pairs (0,6 V/NHE) [6], we obtained an approximate value of the redox potential of the Am(VI)/Am(V) pair. In 1 M NaOH, Ef(Am(VI)/Am(V)) was estimated at about 0,7 V/NHE.
2.5 Pu(VI) disproportionation in concentrated alkaline solutions
At present, it is considered that Pu(VI) (in contrast to Np(VI) and Am(VI)) is stable in alkaline solutions. The disproportionation of Pu(VI) to Pu(V) and Pu(VII) does not take place in a wide interval of NaOH concentrations. This is connected with the high oxidation potential of the Pu(VII)/Pu(VI) pair in alkaline media. It should be noted that the increase of alkali concentration in solution leads to the decrease of the formal potential of the Pu(VII)/Pu(VI) pair, as well as for Np(VII)/Np(VI) [7] and that the difference of the above-mentioned potentials is constant and equal to 270 mV. Besides, the dependence of the Ef(Pu(VII)/Pu(VI)) value to [NaOH] in the 2–12 M interval can be expressed by an empirical equation with the same coefficient as in the case of neptunium(VII): Ef(Pu(VII)/Pu(VI)) = 0,874 – 0,0355 [NaOH] [7]. The assumption that the above-mentioned dependence may be used for calculations of Ef(Pu(VII)/Pu(VI)) in more concentrated alkaline solutions makes it possible to conclude that Ef(Pu(VII)/Pu(VI)) may be close to Ef(Pu(VI)/Pu(V)) in ~20–22 M NaOH. According to our opinion, the disproportionation reaction 2 Pu(VI) = Pu(VII) + Pu(V) is possible under certain conditions, but it requires experimental confirmation.
In our work, the precipitate of PuO3·H2O (~1,5 mg), washed two times with bidistillate water, was treated with hot saturated NaOH solution in the minimum volume by stirring. The contact time of PuO3·H2O with alkali was ~1–2 min. Then, the precipitate was isolated from the alkaline solution and was treated with 3 ml of 5 M LiOH. After centrifugation, the lithium alkali solution turned to a slight blue color. As it is shown in Fig. 4, the spectrum of this solution has a wide absorption band at 550–700 nm, with maximums at 605 and 630 nm, characteristic of Pu(VII). The absorbance of the spectrum of solution in the interval 400–800 nm decreases for more than two times during 2 h. The maximums at 605 and 630 nm became slightly distinguished, that is the evidence of the Pu(VII) reducing in the initial solution. Calculation of Pu(VII) yield leads to a value equal to 20% of the total amount of plutonium, transferred in 5 M LiOH solution. However, the real Pu(VII) yield may be significantly higher than the value obtained, as it is known that very fast Pu(VII) to Pu(VI) reduction occurs under dissolution of solid Pu(VII) compounds in aqueous solutions of alkalis because of large local Pu(VII) concentration at the interface between phases. During the first 2–3 min, up to 50% of Pu(VII) can reduce and then the reduction significantly slows down. Subsequently, it is planed to develop a technique for the determination of the yield of the products of Pu(VI) disproportionation in alkaline media. The appliance of the technique will allow us to establish the dependence of Pu(VII) yield on the alkali concentration in the reaction under investigation.

Absorption spectrum of the interaction product of solid PuO3·x H2O and of a concentrated NaOH solution at 60 °C.
3 Conclusion
It was found that, in high alkaline solutions, a number of Am(III)–(VII) species in oxidized media were stabilized. Of interest is that the redox potentials are changed so dramatically by alkaline solutions that alpha-particle self-irradiation was unimportant. Instead, it was found that Am(VI) is readily reduced by the simple action of water. This is a marked departure from the behavior of Am in other media, where alpha-particle self-irradiation generates redox active species that affect the Am oxidation state [8]. Other redox agents, notably Fe(CN)63– (present in tank wastes), were shown to oxidize Am(III) to Am(IV), with subsequent precipitation as relatively insoluble Am(OH)4. In acidic and near-neutral solutions, Am(IV) is unstable, so this observation is quite important, and demonstrates that redox agents in the tanks will have a definite effect on the americium oxidation state, and hence on the overall solubility of this element. Our results have identified a number of important reactions affecting the oxidation state and the stability of Am in alkaline wastes. Such information will be invaluable to improved separation approaches during remediation. The behavior of Pu in high alkaline solution needs additional confirmation to prove the disproportionation of Pu(VI) into Pu(V) and Pu(VII).
Acknowledgements
The work was supported by the US DOE-OBES, Project RC0-20004-SC14.


