1 Introduction
Tetra-azamacrocycles, in particular derivatives of 1,4,7,10-tetra-azacyclododecane (cyclen) and 1,4,8,11-tetra-azacyclotetradecane (cyclam) [1–6], have a wide variety of real and possible applications [1–10]. One significant medical application is that of bis(macrocyclic) species as anti-viral agents [11–13]. Although various methods for the synthesis of aggregates of macrocycles have been developed [11–15], these have not been applied commonly to precursors possessing pendent functional groups, which might subsequently be used as sites for attachment of these aggregates to proteins or other biopolymers [16]. As part of a programme of work concerned specifically with the synthesis of ‘externally’ functionalised macrocycles [17], we have prepared the bis(hydroxymethyl)-functionalised tetramine 1 [18] and find that it can be efficiently converted to a bis(macrocycle) (2) complex through a familiar template reaction [1,2] based on aminal formation (Scheme 1). To confirm the exact nature of this material, obtained as its paramagnetic Cu(II) complex, we have determined its crystal structure, and report the results of this and the synthetic procedures herein.

Preparation of a tetrakis(hydroxymethylbis(macrocycle).
2 Experimental
2.1 Synthesis
A solution of [Cu(1)]Cl2 (1.0 g) in methanol(150 ml) [18] was mixed with ethane-1,2-diamine (0.1 g), formaldehyde (35%, 5 ml) and triethylamine (1.0 g), then heated at reflux for 2 days. Cooling to room temperature resulted in the formation of a pink precipitate, which was collected by filtration, washed with ethanol (3 × 10 ml) and then dried under vacuum. Yield, 0.4 g (19%). The chloride salt was dissolved in water (10 ml), NaClO4 added and the solution allowed evaporating slowly to provide crystals suitable for a structure determination. Analysis for Cu2(2)(ClO4)2 = C24H56Cl4Cu2N10O20, found (calc.) C, 27.1 (26.85); H, 5.3 (5.26); N, 13.2 (13.05)%.
2.2 Crystallography
Intensity data at 293(2) K for [Cu2(2)(OClO3)4] were collected on an Enraf–Nonius CAD4 four-circle diffractometer using graphite monochromated Mo Kα radiation (λ 0.71073 Å) in the ω–2θ scan mode. Lattice dimensions were determined by a least-square fit of the setting parameters of 25 independent reflections. Data reduction and empirical absorption corrections (ψ-scans) were performed with the WINGX package [19]. The structure was solved by the heavy-atom method with SHELXS and refined by full matrix least-square analysis with SHELXL97 [20]. All non-H atoms were refined with anisotropic thermal parameters, and H-atoms were constrained at estimated positions. The hydroxymethyl groups were disordered over two equally populated sites.
[Cu2(2)(OClO3)4] = C24H56Cl4Cu2N10O20; Mr 1073.67; monoclinic, space group P21/n; a 8.3530(7), b 14.656(3), c 16.598(2) Å, b 95.79(1)°; V 2065.4(5) Å3; F(000) 1112. Dcalc (Z = 2) 1.726 Mg m–3; μMo 1.378 mm–1; specimen 0.50 × 0.20 × 0.20 mm; Nt 3880, Nobs (I > 2 σ(I)) 3614 (Rint 0.0187); R1 0.036, wR2 0.087.
Supplementary information: crystallographic data, in the form of a .cif file, has been deposited with the CCDC, deposition number 239845. These data may be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; Fax. (+44) 1223-336-033 or deposit@ccdc.cam.ac.uk.
3 Results and discussion
The anti-viral activity of the Zn(II) complex of the bis(macrocycle), p-xylylbis(cyclam), which greatly exceeds that of other metal complexes of this ligand, has been analysed [13] in terms of the presence of ligand conformations other than the trans-III configuration found most commonly with cyclam complexes [17a,21], the rapidity of conversion between several configurations, and the capacity of Zn(II) to bind to carboxylate entities such as are present in side chains of HIV-protease. The fact that the bridge between the two cyclam units of this molecule is attained by N-alkylation, which converts one secondary N of each cyclam to a tertiary N, has structural consequences in elongating one Zn–N bond [13], an effect seen in various related systems [22], and may explain the configurational lability of the complex. In several senses, therefore, the present complex may prove useful for comparison.
The lattice of Cu2(2)(ClO4)4 can be regarded as if made up from centrosymmetric molecular units [Cu2(2)(OClO3)4] (Fig. 1), each Cu(II) centre having a tetragonally distorted N4O2 coordination environment. Monodentate binding of perchlorate to Cu may be assisted by intramolecular H-bonding of a second oxygen to NH. Notwithstanding Jahn–Teller effects resulting in axial elongation of the coordination sphere in most six-coordinate Cu(II) complexes, given that monodentate binding to Cu(II) is expected to be of similar lability to that to Zn(II) [23], clearly this bis(macrocycle) complex should share with the Zn(II)-xylylbis(cyclam) species the ability to bind to protein carboxylate entities. While the ligand conformation in the Zn species only might permit carboxylate chelation, the H-bonding possible in the present Cu species would enable two-site binding of carboxylate. This H-bonding reflects the NH orientation possible in the given ligand conformation, the present complex being similar to many others formed from cyclam ligands substituted on the central carbon of the trimethylene link [17] in that the functionalisation appears to result in minimal perturbation of the coordination characteristics of the parent ligand (despite fairly marked variations in the orientations of the N-pendent substituents, which in the present case are close to axial). Thus, each bound macrocycle has the trans-III configuration, with all Cu–N bond lengths closely similar (Table 1).
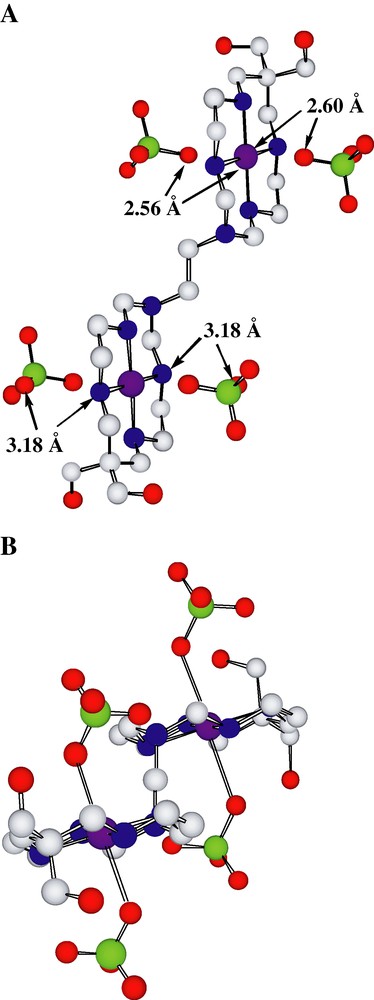
(A) The molecular unit of the lattice of [Cu2(2)(OClO3)4]. For clarity, only one component of the disorder involving the hydroxy substituents is shown. Atom separations indicating perchlorate-O coordination to Cu and H-bonding to NH are shown. (B) The same unit viewed down the C–C bond of the ethylene bridge, showing the trans disposition of the large substituents. (Coordinate bonds to perchlorate shown.).
Selected bond lengths (Å) and angles (°) for [Cu2(2)(OClO3)4]
| Cu(1)–N(3) | 1.999(3) |
| Cu(1)–N(1) | 2.008(3) |
| Cu(1)–N(2) | 2.017(3) |
| Cu(1)–N(4) | 2.019(3) |
| N(3)–Cu(1)–N(1) | 178.95(13) |
| N(3)–Cu(1)–N(2) | 93.67(11) |
| N(1)–Cu(1)–N(2) | 86.51(12) |
| N(3)–Cu(1)–N(4) | 85.72(11) |
| N(1)–Cu(1)–N(4) | 94.08(12) |
| N(2)–Cu(1)–N(4) | 179.06(11) |
An interesting feature is the presence of short perchlorate-O…perchlorate-O contacts ~2.76 Å, a distance which has been interpreted in various metal carbonate structures as indicative of attractive O···O interactions [24]. Disorder of the hydroxymethyl group orientations may be a consequence of the multiple possibilities for H-bonding to or by these units indicated by intramolecular OH···OH, intermolecular OH···O(perchlorate), and inter- and intra-molecular O(H)···N(H) separations between 2.9 and 3.2 Å. The binuclear complex cation can be regarded as providing H-bond donor and acceptor sites separated by ~20 Å. The disposition of the two large functional groups on the central ethylene bridge is trans. In the absence of solution structural information at this point for this complex, no simple comparison can be drawn with the group of macrocyclic but non-metal-complex anti-viral agents [25,26] known to bind to their target enzyme through multiple H-bonding interactions.
If complexes such as that presently described do prove to have anti-viral activity, the advantage they offer over other bis(macrocycles) is their ease of synthesis. In principle the aminal (N–CH2–N) groups at the bridgehead of each macrocycle present stability problems for the metal-free ligand, but Cu(II)-to-Zn(II) transmetallation should be possible by using procedures successfully employed for structurally similar tetraamine macrocycles [27]. At present, we are investigating extensions of the synthesis to provide asymmetric bis(macrocycles) involving mixed donor atoms and bridges of varying lengths.
Acknowledgement
This work was supported by the Brain Busan 27 Project in 2003.


