1 Introduction
The study of heteropolychalcogenide anions has developed largely during the last decade [1–7]. In particular, the Te/Se system includes the [TeSe2]2– [4], [TeSe3]2– [2,4], [Te(Se5)2]2– [8], [Te(Se5)3]4– [9], [{Te(Se2)2}2(μ2-(Se2)]2– [9], and [Te3Se6]2– [10] anions. Some of these anions should be able to ligate metal systems. Surprisingly, there are only a few such examples known. These include the species [Hg(Te2Se2)2]2– [11], [Au(TeSe2)]22– [12], [Au(TeS3)]22– [13], and [(Ag(TeQ3))2Te]2– (Q = S, Se) [13]. Here we describe the preparation and structural characterization of the new compounds [PPh4][(CpM(μ2-Se2))3(μ3-O)(μ3-TeSe3)] (M = Zr, Hf), in which TeSe32– groups act as tridentate ligands to form three M–Se bonds.
2 Preparation
2.1 General procedures
All experiments were carried out under an N2 atmosphere with the use of Schlenk-line techniques. Na2[TeSe3] was synthesized by the reaction of stoichiometric quantities of the elements in liquid NH3. Te powder and Se powder were purchased from Aldrich Chemical Co., Milwaukee, Wisconsin and Cerac, Inc., Milwaukee, Wisconsin, respectively, and used as received. Cp2ZrCl2 and Cp2HfCl2 were purchased from Strem Chemicals, Inc., Newburyport, Maine. Anhydrous Et2O from Fisher Chemicals, Inc., Fair Lawn, NJ was dried over Na/benzophenone; N,N-dimethylformamide (DMF) from Fisher Chemicals, Inc. was dried over molecular sieves.
2.2 Synthesis of [PPh4][(CpM(μ2–Se2))3(μ3–O)(μ3–TeSe3)] (M = Zr, Hf)
Na2[TeSe3] (100 mg, 0.26 mmol) was dissolved in 5 ml of DMF. To this brown solution 88 mg (0.30 mmol) of solid Cp2ZrCl2 or 115 mg (0.30 mmol) of Cp2HfCl2 was added. The resulting solution was stirred under an N2 atmosphere for 5 h. Then 126 mg (0.30 mmol) of solid [PPh4]Br was added to the solution. The solution was stirred for an additional hour and then filtered through a cannula. Next 3 ml of this solution was transferred into a glass tube (5 mm diameter) that had been evacuated and filled with N2. Then the solution was carefully layered with 5 ml of Et2O and the tube was sealed with a rubber septum and parafilm. In 5 days, several orange crystals of [PPh4][(CpM(μ2-Se2))3(μ3-O)(μ3-TeSe3)] (M = Zr, Hf), suitable for X-ray diffraction studies, were obtained. This synthesis was subsequently repeated several times to afford the same products.
3 X-ray structure determinations
Single-crystal X-ray diffraction data were collected with the use of graphite-monochromatized Mo K α radiation (λ = 0.71073 Å) at 153 K on a Bruker Smart-1000 CCD diffractometer [14]. The crystal-to-detector distance was 5.023 cm. Crystal decay was monitored by recollecting 50 initial frames at the end of data collection. Data were collected by a scan of 0.3° in ω in four sets of 606 frames at φ settings of 0, 90, 180, and 270°. The exposure times were 15 s per frame. The collection of the intensity data was carried out with the program SMART [14]. Cell refinement and data reduction were carried out with the use of the program SAINT [14] and face-indexed absorption corrections were performed numerically with the use of the program XPREP [15]. Then the program SADABS [14] was employed to make incident beam and decay corrections.
The structures were solved with the direct methods program SHELXS and refined with the full-matrix least-squares program SHELXL of the SHELXTL suite of programs [15]. Hydrogen atoms were generated in calculated positions and constrained with the use of a riding model. Additional details may be found in Section 5.
4 Results and discussion
Reaction of Na2[TeSe3] with Cp2ZrCl2 or Cp2HfCl2 in DMF followed by slow addition of Et2O afforded orange plates of [PPh4][(CpZr(μ2-Se2))3(μ3-O)(μ3-TeSe3)] or [PPh4][(CpHf(μ2-Se2))3(μ3-O)(μ3-TeSe3)], respectively. The yields of these compounds were too low to enable chemical or spectroscopic analyses to be performed. Consequently, their characterization rests entirely on the single-crystal structural analyses. The μ3 capping atom was assigned to O because of the known oxophilicity of Zr and Hf, the resultant sensible M–O distances, and the reasonable displacement parameters. Thus, we believe there is a μ3-O cap present in both structures even though the reactions were carried out in the presumed absence of a source of oxygen, other than Et2O. Attempts have been made to increase the yields of these compounds by introducing an oxygen source, such as O2, water, hydrogen peroxide, or m-chloroperoxybenzoic acid, into the syntheses. These attempts were unsuccessful. Moreover, when Et2O was added under anaerobic conditions and the reaction flask was sealed with a glass stopper no product was obtained. Thus, we have no explanation for the source of the oxygen. However, it is not unusual to have adventitious sources of oxygen produce novel chemical compounds [16–21].
Selected crystallographic data are presented in Table 1. The structure of [PPh4][(CpM(μ2-Se2))3(μ3-O)(μ3-TeSe3)] (M = Zr, Hf) comprises well-separated cations and anions. The metrical features of the cation are normal. The [(CpZr(μ2-Se2))3(μ3-O)(μ3-TeSe3)] – anion is depicted in Fig. 1 and the structure of its [(Zr(μ2-Se2))3(μ3-O)(μ3-TeSe3)] core is shown in Fig. 2. Selected bond distances and angles for both compounds are summarized in Table 2. The structure of the anion comprises a triangle of what are formally MCp3+ groups bridged by three μ2-Se22– ligands and capped by a μ3-TeSe32– and a μ3-O2– ligand. The mean M···M distances of about 3.56 Å are longer than those found in other M3 or M6 clusters (range 3.22–3.52 Å) where M–M bonds have been assigned [22,23]. The capping O atom lies above the M3 plane by 0.441 Å (M = Zr) or 0.422 Å (M = Hf). The (μ3-O)M3 unit is a common one [24–34]. In these compounds the range of Zr–O distances in the (μ3-O)Zr3 core is 2.004–2.336 Å and the range of Hf–O distances in the (μ3-O)Hf3 core is 2.018–2.086 Å; thus, the M–O distances in Table 2 are typical. Also typical are the M–Se distances involving the μ2-Se2 ligands and the Se–Se distances in those ligands. As can be seen from Table 2, in general the M–Se(Te) distances to the TeSe3 capping groups are longer than are the M–(μ2-Se2) distances.
Selected crystallographic data for [PPh4][(CpZr(μ2-Se2))3(μ3-O)(μ3-TeSe3)] and [PPh4][(CpHf(μ2-Se2))3(μ3-O)(μ3-TeSe3)]
| Zr | Hf | |
| Formula | C39H35OPSe9TeZr3 | C39H35Hf3OPSe9Te |
| Formula weight | 1662.54 | 1924.35 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/n | P21/n |
| a (Å) | 11.1427(9) | 11.1457(16) |
| b (Å) | 29.041(2) | 29.092(4) |
| c (Å) | 14.5717(12) | 14.642(2) |
| β (°) | 111.1600(10) | 111.333(2) |
| V (Å3) | 4397.5(6) | 4422.5(11) |
| T (K) | 153 | 153 |
| Z | 4 | 4 |
| ρcalc (g cm–3) | 2.511 | 2.89 |
| μ (MoKα) (mm–1) | 8.866 | 15.146 |
| R1(Fo) (Fo2 > 2 σ(Fo2))a | 0.0432 | 0.039 |
| wR(Fo2)b | 0.081 | 0.091 |
a
b
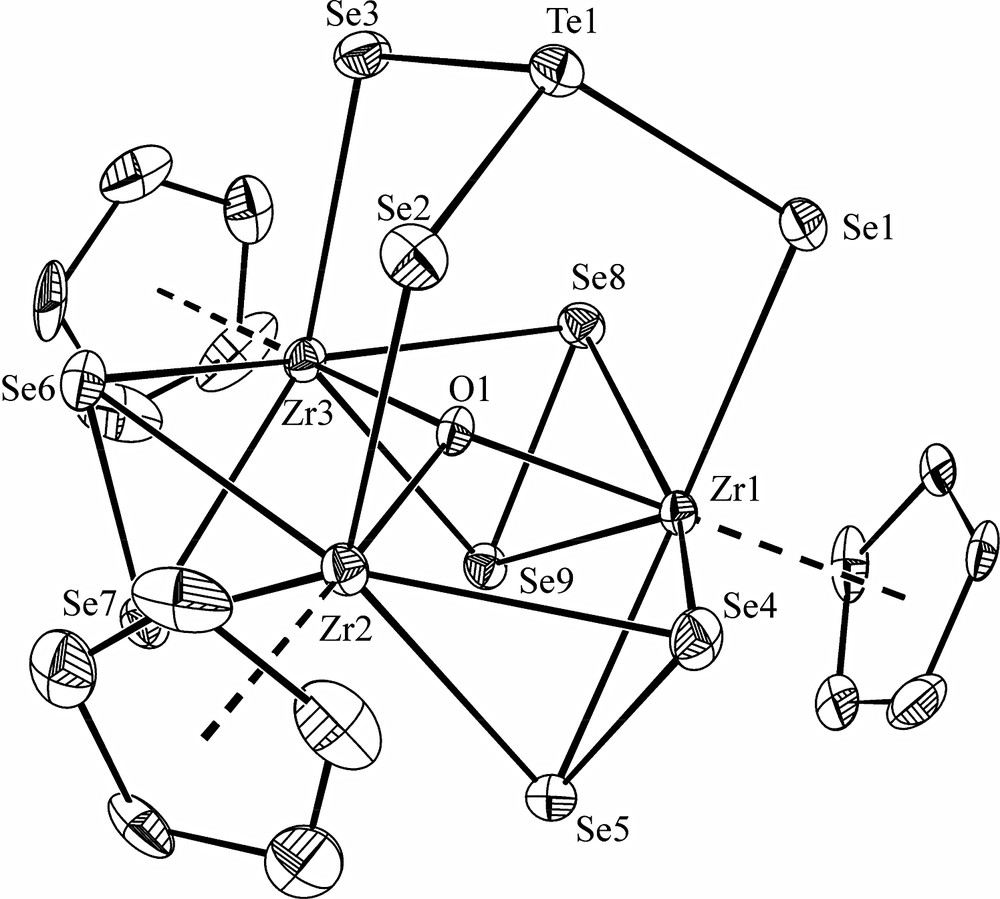
Structure of the anion of [PPh4][(CpZr(μ2–Se2))3(μ3–O)(μ3–TeSe3)]. The displacement ellipsoids are shown at the 50% probability level. Hydrogen atoms are omitted for clarity.
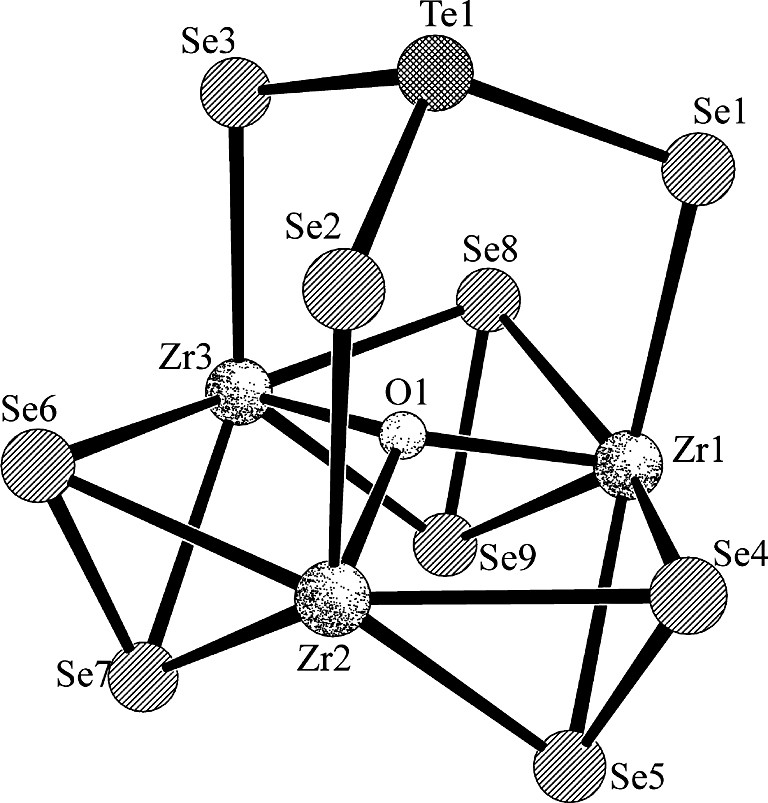
Sketch of the [(Zr(μ2–Se2))3(μ3–O)(μ3–TeSe3)] core of [PPh4][(CpZr(μ2–Se2))3(μ3–O)(μ3–TeSe3)].
Selected bond distances (Å) and angles (deg) for [PPh4][(CpZr(μ2-Se2))3(μ3-O)(μ3-TeSe3)] and [PPh4][(CpHf(μ2-Se2))3(μ3-O)(μ3-TeSe3)]
| Zr | Hf | ||
| Zr(1)···Zr(2) | 3.5604(10) | Hf(1)… Hf(2) | 3.5610(6) |
| Zr(1)···Zr(3) | 3.5760(10) | Hf(1)… Hf(3) | 3.5452(6) |
| Zr(2)···Zr(3) | 3.5677(10) | Hf(2)…Hf(3) | 3.5499(6) |
| Zr(1)–O(1) | 2.099(5) | Hf(1) –O(1) | 2.093(5) |
| Zr(2)–O(1) | 2.112(4) | Hf(2) –O(1) | 2.098(5) |
| Zr(3) –O(1) | 2.109(4) | Hf(3) –O(1) | 2.090(5) |
| Te(1) –Se(1) | 2.4868(9) | Te(1) –Se(1) | 2.4915(10) |
| Te(1) –Se(2) | 2.4867(9) | Te(1) –Se(2) | 2.4799(11) |
| Te(1) vSe(3) | 2.4809(9) | Te(1) –Se(3) | 2.4912(10) |
| Se(1) –Zr(1) | 2.8089(10) | Se(1) –Hf(1) | 2.7871(9) |
| Se(2) –Zr(2) | 2.8059(10) | Se(2) –Hf(2) | 2.7744(9) |
| Se(3) –Zr(3) | 2.7953(11) | Se(3) –Hf(3) | 2.7850(9) |
| Se(4) –Zr(1) | 2.7833(10) | Se(4) –Hf(1) | 2.7472(10) |
| Se(4) –Zr(2) | 2.7778(10) | Se(4) –Hf(2) | 2.7582(10) |
| Se(5) –Zr(1) | 2.7706(11) | Se(5) –Hf(1) | 2.7569(9) |
| Se(5) –Zr(2) | 2.7563(10) | Se(5) –Hf(2) | 2.7662(9) |
| Se(6) –Zr(2) | 2.7900(10) | Se(6) –Hf(2) | 2.7609(9) |
| Se(6) –Zr(3) | 2.7737(10) | Se(6) –Hf(3) | 2.7770(9) |
| Se(7)–Zr(2) | 2.7641(10) | Se(7) –Hf(2) | 2.7493(9) |
| Se(7)–Zr(3) | 2.7584(10) | Se(7) –Hf(3) | 2.7507(9) |
| Se(8) –Zr(1) | 2.7611(10) | Se(8) –Hf(1) | 2.7672(10) |
| Se(8) –Zr(3) | 2.7702(11) | Se(8) –Hf(3) | 2.7670(9) |
| Se(9) –Zr(1) | 2.7728(10) | Se(9) –Hf(1) | 2.7606(9) |
| Se(9) –Zr(3) | 2.7751(10) | Se(9) –Hf(3) | 2.7467(9) |
| Se(4) –Se(5) | 2.3464(11) | Se(4) –Se(5) | 2.3637(12) |
| Se(6) –Se(7) | 2.3601(10) | Se(6) –Se(7) | 2.3704(11) |
| Se(8) –Se(9) | 2.3507(11) | Se(8) –Se(9) | 2.3544(12) |
| Se(2) –Te(1) –Se(1) | 107.40(3) | Se(2) –Te(1) –Se(1) | 107.12(4) |
| Se(3) –Te(1) –Se(1) | 107.29(3) | Se(3) –Te(1) –Se(1) | 107.11(4) |
| Se(2) –Te(1) –Se(3) | 108.35(3) | Se(2) –Te(1) –Se(3) | 107.96(4) |
These compounds represent the first examples of the μ3-TeSe32– ligand. As would be expected, the Te–Se distances within the TeSe3 cap are slightly longer than those reported for the [TeSe3]2– anion in [K{2,2,2-crypt}][TeSe3] (2.454(4) –2.465(4) Å) [4]. We anticipate that the [TeSe3]2– anion will be a useful capping ligand for trinuclear metal clusters in addition to those of Zr and Hf.
5 Supplementary material available
Crystallographic data in CIF format for [PPh4][(CpZr(μ2–Se2))3(μ3-O)(µ3-TeSe3)] (CCDC 238937) and [PPh4][(CpHf(μ2-Se2))3(μ3-O)(µ3-TeSe3)] (CCDC 238938). This material is available free of charge from Cambridge Crystallographic Data Center (CCDC), 12 Union Road, Cambridge, CB2 1EZ, UK. Tel.: +44-1223-336408; fax: +44-1223-336033. E-mail: data_request@ccdc.cam.ac.uk.
Acknowledgments
This work was supported in part by the US National Science Foundation under Grant CHE-9819385. This work made use of Central Facilities supported by the MRSEC program of the National Science Foundation (DMR00-76097) at the Materials Research Center of Northwestern University.


