1 Introduction
Among the large number of molecular-based magnets reported to date [1–3], these containing two transition metal ions [4–8] linked by an organic spacer have suscitated a lot of interest, principally due to the unusual magnetic properties, that may arise from bimetallic magnetic cooperativity.
In these compounds, not only the nature of the ions but also the structure of the organic spacer is of crucial importance. Depending on its geometry and shape, different magnetic interactions will take place between the neighboring metal centers.
In this respect bis-oxamide ligands have been extensively investigated. Following simple synthetic protocols, these ligands allow tight association of different metal centers belonging to the d-block elements in hetero-bimetallic materials.
Such ligands were initially synthesized by Nonoyama and Ojima [9] and have recently been demonstrated by Pey et al. [10] to constitute very promising spacers for elaboration of magnetic molecular-based materials. Indeed in (Gd3+–Cu2+) model complexes containing 4f–3d pairs, it was established that the Gd3+–Cu2+ interaction was usually ferromagnetic [11–14].
Using the bis-oxamide unit as spacer [15–20] it has been subsequently shown that extended linear compounds could retain long range ferromagnetic orderings at low temperature [21]. It was then further suggested that by increasing the dimensionality of the final material, i.e. by strengthening interchain or interplan interactions, magnetic materials resulting from cooperative ferromagnetic interactions may be formed.
In a simple approach, this may be achieved by use of a bis-oxamide ligand possessing an additional coordination site which would be able to bring the metal centers belonging to different poly-bis-oxamide chains close to each others in the solid state (Scheme 1).
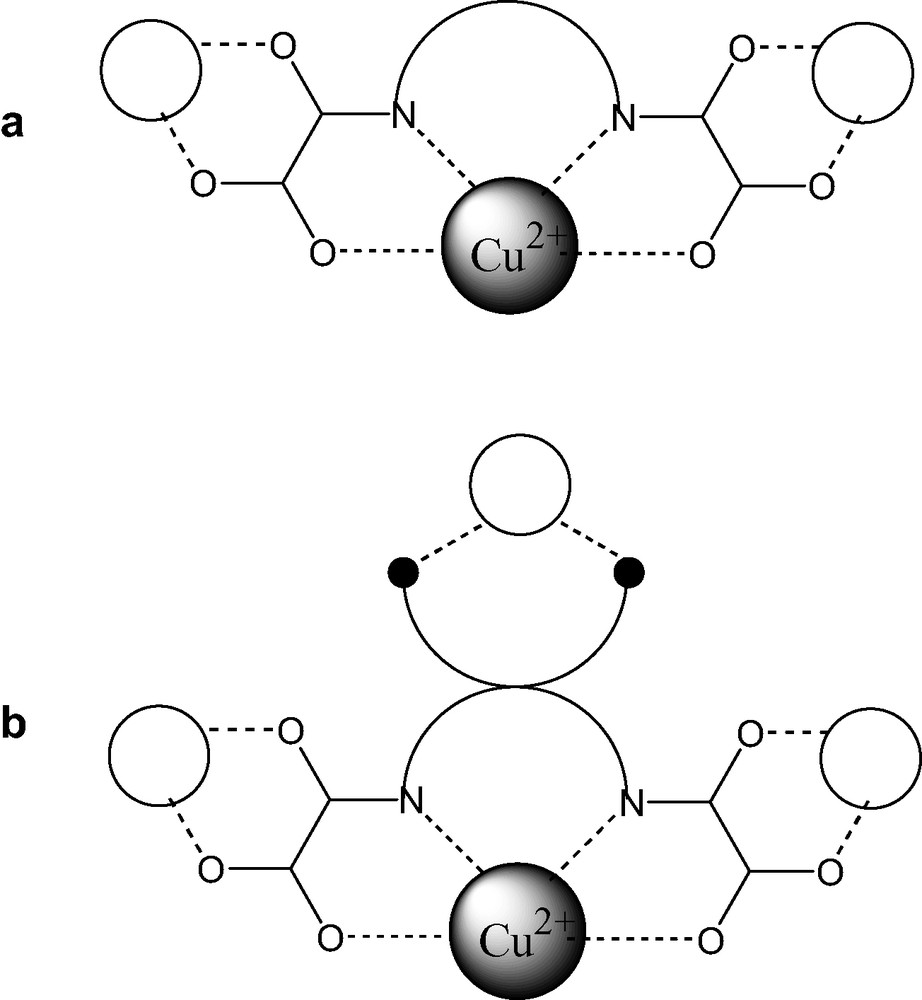
We therefore report in the following (i) the synthesis of such a functional bis-oxamide ligand; (ii) the characterization of its copper complex and (iii) preliminary attempts to use it as a molecular precursor for synthesis of heterometallic Gd–Cu materials. The solid state magnetic properties of such a material are however very difficult to predict and obviously will depend of the nature of the metal used as much as of solid state structure of the resulting material.
2 Experimental part
2.1 General remarks
All reactions were performed under argon and were magnetically stirred. Solvents were distilled from appropriate drying agent prior to use, THF was distilled over sodium. Commercially available reagents were used without further purification unless otherwise stated. Melting points were determined on Kofler Bank apparatus. IR spectra : ATI Mattson, Genesies series FTIR spectrophotometer, on KBr pellets. NMR spectra: Bruker-ARX 400 spectrometer; δ(H) in ppm rel. to residual protiated solvent CD3OD (3.30), δ(C) in ppm rel. to residual protiated solvent CD3OD (49.02). Mass spectrometry analyses were effected at the ‘Centre régional de mesures physiques de l’Ouest’ (CRMPO) on a high resolution MS/MS ZabSpec TOF Micromass spectrometer. Elemental analyses (C, H, N) were performed by the analytical service of the CNRS at Vernaison and Cu, Na and Gd were performed by JY24 (JOBIN et YVON) ICP spectrometer. X-ray powder pattern were obtained on a RIGAKU D-MAXII diffractometer.
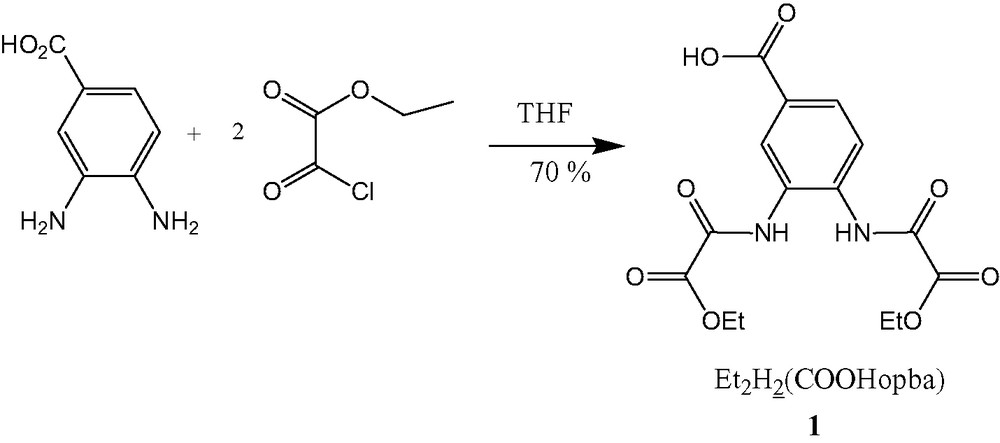
2.2 Preparation of diethyl ester Et2H2(COOHopba), compound 1
A 7-ml (8.18 g, 0.06 mol) sample of a solution of ethyl oxalyl chloride was added dropwise to a stirred mixture of 4.60 g (0.03 mol) of diamino benzoic acid in 200 ml of THF, heated at 50 °C. The resulting suspension was refluxed for 1 h and then filtered to eliminate the solid residue. The THF solution was subsequently concentrated and the resulting red violet solution was allowed to stand for 12 h at room temperature. A pale violet powder then formed. This powder was filtrated, washed with water, and dried under vacuum. Yield: 7.11 g (67%). M.p.: 199 °C. IR (KBr) : 1760 cm–1 (carboxylic acid); 1726 cm–1 (sh), 1718 cm–1, 1705 cm–1 (sh), 1696 cm–1 (amides and esters). Anal. Calc. for Et2H2(COOHopba)·H2O (1) or C15H18N2O9: C, 48.65; H, 4.90; N, 7.56. Found C, 48.99; H, 4.77; N, 7.03. 1H-NMR (CD3OD): 1.42 (m, 3J = 7.1 Hz, 6H, CH3); 4.42 (m, 3J = 7.1 Hz, 4H, CH2); 7.89 (d, 3J = 8.5 Hz, 1H); 8.00 (dd, 3J = 8.5 Hz, 1J = 1.7 Hz, 1H); 8.27 (d, 1J = 1.7 Hz,1H). 13C-NMR (CD3OD): 14.2 (2Cp, 2CH3); 64.4 (2Cp, 2CH2); 125.7; 128.4; 129.4 (3Ct); 130.3; 135.3; 157.3; 157.8; 164.0; 168.4 (6C). X-ray powder spectrum: Table 1.
X-ray powder pattern and refractive index measurement of compound 1
| d (Å) | I |
| 20.422 | 54 |
| 10.052 | 84 |
| 9.507 | 100 |
| 7.323 | 42 |
| 6.622 | 8 |
| 6.016 | 13 |
| 5.653 | 26 |
| 5.349 | 75 |
| 5.167 | 7 |
| 4.773 | 8 |
| 4.514 | 22 |
| 4.159 | 12 |
| 3.777 | 13 |
| 3.727 | 16 |
| 3.643 | 96 |
| 3.497 | 9 |
| 3.424 | 27 |
| 3.267 | 57 |
| 3.155 | 8 |
| 3.097 | 14 |
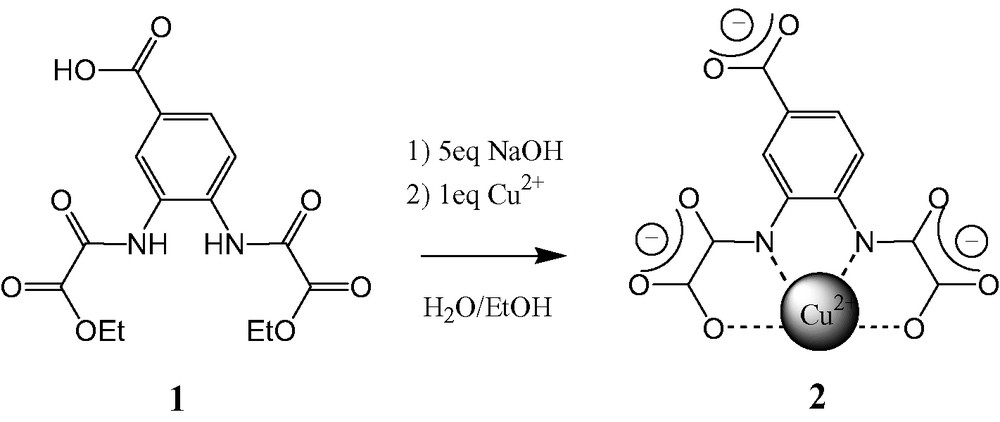
2.3 Preparation of copper(II) precursor salt Na3[Cu(COOopba)]·3 H2O, compound 2
A 25-ml sample of an aqueous solution containing 1.64 g (0.041 mol) of NaOH was added to a suspension of 2.65 g (0.0075 mol) of Et2H2(COOHopba) in 50 ml of a 9:1 mixture of water/ethanol. The mixture was heated at 60 °C for 0.5 h, then 25 ml of an aqueous solution containing 1.82 g of Cu(NO3)2·3H2O (0.0075 mol) were added. The black solution was filtered, the water solution was concentrated and the resulting solution was allowed to stand for 12 h at room temperature. The dark violet powder obtained was collected by filtration. Yield: 2.76 g (79%). Recrystallization from a water/ethanol mixture gave dark violet needles. IR (KBr): 1671 cm–1 (sh), 1623, 1583, 1540 cm–1 (amides and carboxylates). FAB-MS negative mode (Matrix glycerol): 399.9 (L5–Cu2+2Na+)–, 377.9 (L5–Cu2+Na+H+)–. Anal. Calc. for Na3[Cu(COOopba)]·3 H2O (2) or C11H9N2O11Cu1Na3: C, 27.66; H, 1.90; N, 5.86; Na, 14.4; Cu, 13.3. Found C, 27.69; H, 1.84; N, 5.91; Na, 13.3; Cu, 12.2. X-ray powder spectrum made on 20 mg of dark violet needles: Table 2.
X-ray powder pattern and refractive index measurement of compound 2
| d (Å) | I |
| 12.733 | 26 |
| 10.778 | 27 |
| 10.057 | 100 |
| 7.812 | 17 |
| 6.956 | 19 |
| 6.059 | 25 |
| 5.686 | 9 |
| 4.927 | 23 |
| 4.606 | 11 |
| 3.901 | 17 |
| 3.507 | 9 |
| 3.404 | 45 |
| 3.313 | 14 |
| 3.201 | 16 |
| 3.227 | 16 |
| 3.134 | 14 |
| 3.097 | 35 |
| 2.791 | 43 |
| 2.598 | 12 |
| 2.247 | 15 |
2.4 Preparation of copper(II)–gadolinium(III)-based material, compound 3
120 mg of water soluble copper(II) complex 2 (0.25 mmol) in 10 ml of water are added to 93 mg of GdCl3·7 H2O (0.25 mmol) in 10 ml of water, immediately a dark blue green precipitate forms. The powder obtained was collected by filtration and washed thoroughly with water.
IR (KBr): 1636, 1600–1500 cm–1 (amides, carboxylates). Anal. found for material 3: Cu, 8.0; Gd, 22.0; Na, 0.1.
3 Results and discussion
The ligand 1 was synthesized by reacting the diamino benzoic acid with ethyl oxalyl chloride (Scheme 2). This ligand was characterized by elemental analyses, IR and NMR spectroscopies. The elemental analyses show the expected chemical composition for the free ligand with a water molecule present as solvate: Et2H2(COOHopba)·H2O, opba standing for o-phenylenebis-(oxamato). Its 1H-NMR spectrum displays the characteristic AB system due to the aromatic ring protons and a tertiary coupling between the two nearest protons on each side of the carboxylate protons group. The IR spectrum of the free ligand Et2H2(COOHopba)·H2O, further evidences that the carbonyl groups have been preserved since strong carbonyl stretching bands around 1700 cm–1 (amide, carboxylic acid and ester groups) are observed, and confirms the presence of the water solvate in crystals (band near 3500 cm–1).
The corresponding copper complex 2 was obtained in a straightforward manner in two steps i.e. one-pot saponification of the two ester functions followed by reaction of the carboxylate groups in basic medium with the copper(II) salt (Scheme 3). The resulting complex 2 was isolated as a violet powder and characterized by elemental analyses, IR, FAB-MS and X-ray powder diffraction. The elemental analyses show that the copper complex has the expected formula Na3[Cu(COOopba)]·3H2O. The strong carbonyl stretching bands of the free ligand, are shifted by ca. 70 cm–1 to lower wavenumbers. This is characteristic of the deprotonation and the coordination of the carbonyl oxygens in the oxamido unit to the copper metal ion. Finally, the FAB-MS (negative mode) spectrum for Na3[Cu(COO–opba)]·3 H2O exhibits the molecular peak at 399.9 expected for the complex. X-ray powder patterns, using Cu Kα radiation, obtained for the free ligand 1 (Table 1) and for the copper complex 2 (Table 2), indicated that the two solids are well crystallized, with large cell parameters, especially for the free ligand 1.
The charge of the compound 2 is 3– and the copper complex [Cu(COOopba)]3– can be envisioned as a new molecular anionic building block. Indeed, this ‘complex ligand’ has still two free O–O sites, and a free carboxylate site available for the coordination (Scheme 1b). Interestingly, this complex is very water soluble. Accordingly, in order to isolate cross-linked bis-oxamide chains in the solid state, we have tried to link theses units with a lanthanide salt. Lanthanide salts are very oxophillic and should readily complex all oxygen coordination sites available.
Thus, gadolinium chloride and the anion [Cu(COOopba)]3– were reacted in an aqueous solution, in a preliminary test. The copper(II)–gadolinium(III)-based material 3 was formed readily and isolated as a dark precipitate. This precipitate was filtered of and analyzed. Unfortunately, its insolubility in most solvent limited seriously the spectroscopic investigations to uncover its structure. The Cu, Na and Gd analyses indicated that the Cu/Gd ratio is around 1:1 in this material, as expected. Therefore, no sodium is anymore present and the solid has the chemical formula corresponding to an inorganic polymer LnCuGd, L standing for the deprotonated free ligand 1. While an X-ray powder analysis of this precipitate was suggestive of a highly amorphous nature for the material isolated. Attempts to grow crystals of this materials directly from the reaction medium by slow mixture of the reactants were unsuccessful for now and further studies to clarify its structure are currently underway.
Nevertheless, this result indicates clearly that a hetero-bimetallic material can easily be formed from the copper complex 2 by reacting it with lanthanide salts. In the future, we will investigate in more details the structure of this material to proof that the lanthanide ions are really coordinated to the carboxylate groups, and not just act as counter ions.
A most interesting topology for this molecular material would be poly-dimensional netting where f-elements cross link the chains or the layers chemically, by coordination to the carboxylate COO– group.
4 Conclusions
The straightforward synthesis of a new bis-oxamide ligand 1 incorporating a pendant carboxylate group has been described. The corresponding Cu(II) complex 2, can be readily synthesized and has been characterized. This unit constitutes a new building block easily available and presenting three oxygen-based free coordination sites. These can further undergo complexation reactions with lanthanide salts and form readily new molecular-based hetero-bimetallic d–f materials.
5 Supplementary material
The X-ray powder spectra of free ligand 1 and Cu complex 2 are available from the author on request, as well as the IR spectra of compounds 1–3.
Acknowledgements
We specially thank Dr. Yannick Cudennec (GRCM-INSA de Rennes) for kindly help for use of ICP JY24 spectrometer, and helpful discussions.


