1 Introduction
Giant polyoxometalates (POM) are relatively recent but increasingly fascinating object of study. Their peculiar topological, magnetic, optical and electric properties are being thoroughly investigated. One way of modifying their properties (and structure) is to alter self-assembly conditions, which give a specific giant POM molecule [1,2]. Another way to the structural and functional diversity involves using ‘pre-fabricated’ giant POM as macroligands toward heterometals. The heterometal can bring into the play its own specifics, such as magnetism. It also can exercise a structure-directing influence, facilitating the formation of even larger POM or polymeric frameworks. The oxophilic and paramagnetic lanthanide ions are especially well suited for this purpose [3–5]. One shall always keep in mind, however, that fragmentation and re-assembly of giant POM into different structures can also occur during these reactions. Here we report synthesis and structure of [{Gd(H2O)5}4{Mo36(NO)4O108(H2O)16}]·34 H2O (1), where the polyoxomolybdate fragments {Mo36(NO)4O108(H2O)16} formed by a degradation of the keplerate, [{(MoVI)MoVI5O21(H2O)6}12{MoV2O4CH3COO)}30]42–, are bound into a 2D-framework by {Gd(H2O)5} units.
2 Experimental section
2.1 Synthesis of 1
To a stirred solution of Gd(NO3)3·5H2O (0.90 g, 2.08 mmol) in H2O (60 ml), the NH4+ salt of [{(MoVI)MoVI5O21(H2O)6}12{MoV2O4CH3COO)30]42– [6] (1.00 g, 0.036 mmol) was added. The resulting mixture was vigorously stirred in an open 100-ml Erlenmeyer flask (wide-necked) for 24 h. After acidification with HCl (1 M, 3 ml) and addition of NaCl (1.0 g), the stirred reaction mixture was heated to 90–95 °C and then filtered whilst still hot, and kept at 20 °C. After 1.5 months (from time to time separated amorphous solids were removed by filtration) light brown rhombic crystals of 1 were collected by filtration through a glass frit, washed twice with a little iced water, and dried in air. Yield: 0.221 g (23% based on molybdenum).
Elemental analysis for H140Gd4Mo36N4O182 calculated (%):H 1.96; N 0.78; found (%):H 1.85; N 0.83.
IR (KBr pellet, cm–1): 3517 (m); 3448 (m); 3364 (w); 3157 (w); 1617 (m); 1403 (m); 950 (m); 874 (s); 782 (m); 698 (m); 621 (s); 567 (s); 537 (s); 360 (m); 330 (m); 238 (m).
2.2 X-ray structure determination
The crystallographic data for 1 and structure refinement parameters data are listed in Table 1. The structure was solved by direct methods and refined by the full-matrix least-squares technique against P2 with anisotropic displacement parameters for all atoms except for eight oxygen atoms of solvate water molecules. Two of such solvate water molecules were refined with half occupation. Hydrogen atoms were not defined. The SAINT [7] and SHELXTL-97 [8] program packages were used throughout the calculations.
Crystallographic data and details of diffraction experiments for 1.
| Empirical formula | H140Gd4Mo36N4O182 |
| Formula weight | 7192.00 |
| Crystal system | Triclinic |
| Space group | P |
| a (Å) | 13.5035 (14) |
| b (Å) | 16.2894 (17) |
| c (Å) | 18.905 (2) |
| α (°) | 77.376 (2) |
| β (°) | 80.336 (2) |
| γ (°) | 76.042 (2) |
| V (Å3) | 3908.8 (7) |
| Z | 1 |
| Dcalc (g cm–3) | 3.055 |
| Temperature (K) | 120 (1) |
| Difractometr | Bruker SMART 1000 with CCD area detector |
| Radiation | Mo Kα (λ = 0.71073 Å) |
| 2 θmax (°) | 55.60 |
| Range h, k, l | –16 ≤ h ≤ 16, –20 ≤ k ≤ 19, –23 ≤ l ≤ 23 |
| μ (mm−1) | 4.587 |
| Reflections measured | 34 353 |
| Unique reflns | 15 291 |
| Rint | 0.0859 |
| Observed (F0 > 4 σ(F0)) | 6856 |
| Refined parameters | 987 |
| Restraints | 0 |
| R1, wR2 (I > 2 σ(I)) | R1 = 0.0781, wR2 = 0.1599 |
| R1, wR2 (all data) | R1 = 0.1703, wR2 = 0.1848 |
| Goodness-of-fit on F2 | 1.012 |
| (Δρ)max (e Å–3) | 4.543 |
| (Δρ)min (e Å–3) | –4.027 |
3 Results and discussion
Light brown rhombic crystals of 1 are prepared from ammonium salt of keplerate, [{(MoVI)MoVI5O21(H2O)6}12{MoV2O4CH3COO)}30]42– and Gd(NO3)3 in 23% yield by the synthetic procedure described in the Experimental Section. The compound has been characterized by elemental analysis, FT-IR spectroscopy and unambiguously by single-crystal X-ray diffraction technique. The compound 1 and (NH4)12[Mo36(NO)4O108(H2O)16]·36 H2O have similar FT-IR spectra showing only slight shifts in some band positions. The infrared spectra show characteristic absorption bands attributable to H2O, ν(Mo–Oterm), ν(Mo-(μ2-O)) and ν(Mo-(μ3-O)) groups. The ν(NO) vibrational frequency of 1617 cm–1 overlaps with δ(HOH) and is typical for the linear {MoNO}3+ moiety [9].
The structure of 1 was determined by X-ray diffraction from a single crystal, obtained directly in the synthesis. Main bond distances and angles are given in Table 2. The structure is a 2D neutral framework where the POM fragments {Mo36(NO)4O108(H2O)16} [9,10] are united into layers by Gd3+ ions (Fig. 1). The POM anion is build of two large Mo17 subunits held together by two cis-MoO22+ groups. Within each subunit there are two Mo(NO)3+ groups with pentagonal–bipyramidal coordination, surrounded by five MoO6 octahedra: three are derived from MoO4+, and two from cis-MoO22+. These six Mo atoms form a five-pointed star; two such stars are held together by two pairs of edge-sharing octahedra (cis-MoO22+-type), and by one «inner» Mo atom with no terminal oxygen atoms, also octahedrally coordinated. Only terminal groups MoO22+ coordinate Gd3+, and altogether 12 terminal oxygen atoms of [Mo36(NO)4O108(H2O)16]12– participate in this bonding. If we designate the two octahedra, attached to the Mo(NO)3+ unit, as ‘coordinated’, type A; those in the dimer, holding two ‘stars’ together in each subunit, as «periferic», type B, and those holding two Mo17 subunits together, as ‘closing’, type C, we obtain the following pattern of the coordination polymer lattice building: in one direction (along b axis) we have …type A–MoO22+–Gd3+-type C–MoO22+… regularity, while in the other direction (along c axis) this pattern is …type A–MoO22+–Gd3+–type B–MoO22+…. There are two unique gadolinium(III) atoms in the structure of 1. Each Gd3+ ion is octacoordinated. Two neighboring A-type MoO22+ groups behave as bidentate ligands, while those of B- and C-types are monodentate. Thus the coordination polyhedron of each Gd is filled with three POM oxygen atoms, the rest being coordinated water.
Selected bond distances (Å) and angles (°) in 1
| Bond type | Distance (Å) | Angle | Value (°) |
| Gd–O | 2.333(16)–2.49 (2) | Mo–μ4-O–Mo | 95.65 (1)–102.16 (1) |
| 144.76 (1)–146.67 (1) | |||
| Mo–Oterm | 1.662 (17)–1.932 (13) | Mo–μ2-O–Mo | 88.50 (1)–121.25 (1) |
| Mo–OH2O | 2.214 (15)–2.535 (3) | 150.81 (1)–159.06 (1) | |
| Mo–μ2-O | 1.731 (13)–2.398 (13) | Mo–μ3-O–Mo | 91.22 (1)–111.34 (1) |
| 133.53 (1)–144.76 (1 | |||
| Mo-μ3-O | 1.880 (12)–2.275 (12) | Mo–μ2-O–Gd | 152.40 (1)–153.40 (1) |
| 166.30 (1) | |||
| Mo–μ4-O | 2.087 (13)–2.296 (12) | ||
| Mo–N | 1.663 (16)–2.371 (15) | ||
| N–O | 1.22 (2)–1.22 (3) | ||
| Mo–OGdChto eto?? | 1.688 (14)–1.757 (15) |
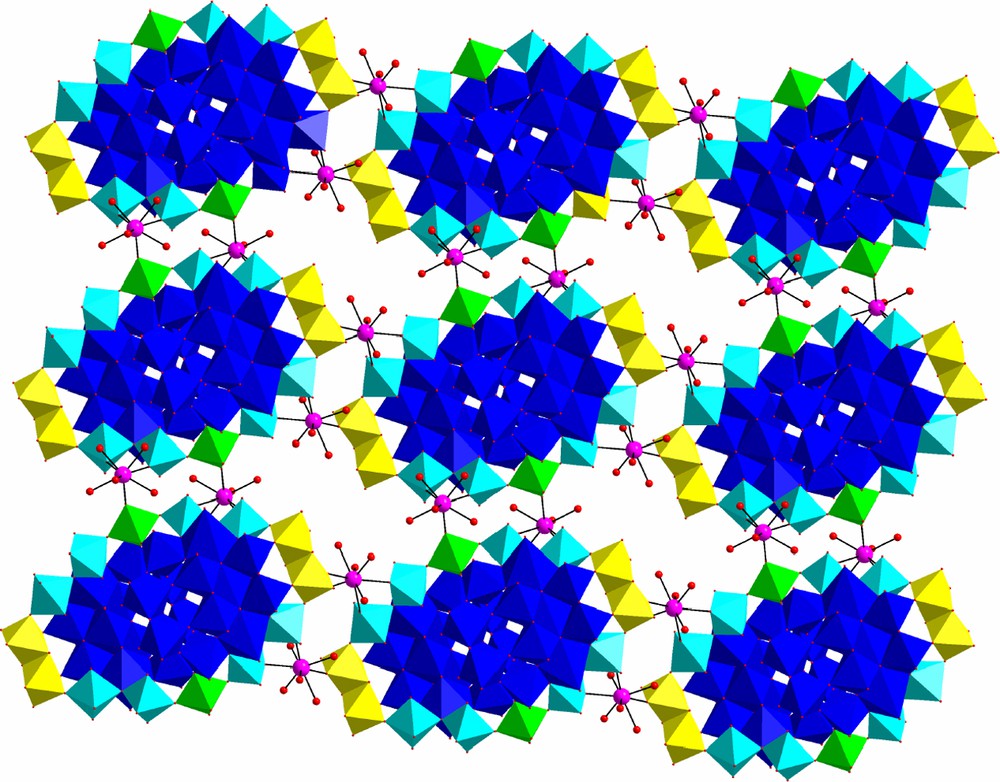
Connectivity pattern in the crystal structure of compound 1 (view along a axis) distinguishing between the building units or constituents: {Gd(H2O)5} fragments in ball-and-stick (Gd-purple; O-red) and {Mo36} fragments in polyhedra (cyan-type A octahedra; yellow-type B octahedra; green-type C octahedra; blue-the rest of polyhedra) representation. Only one layer is shown. Crystallization water molecules are omitted for clarity.
Crystal packing of 1 consists of the adjusted neutral layers stacked in a ABAB… mode (Fig. 2). There are three types of solvent water molecules in 1. Two water molecules are clathrated in the inner cavity of the POM. Some of them are placed inside the layer and others lay in the gap between adjacent layers. There is an extended network of hydrogen bonds involving water molecules and oxygen atoms of the cluster.
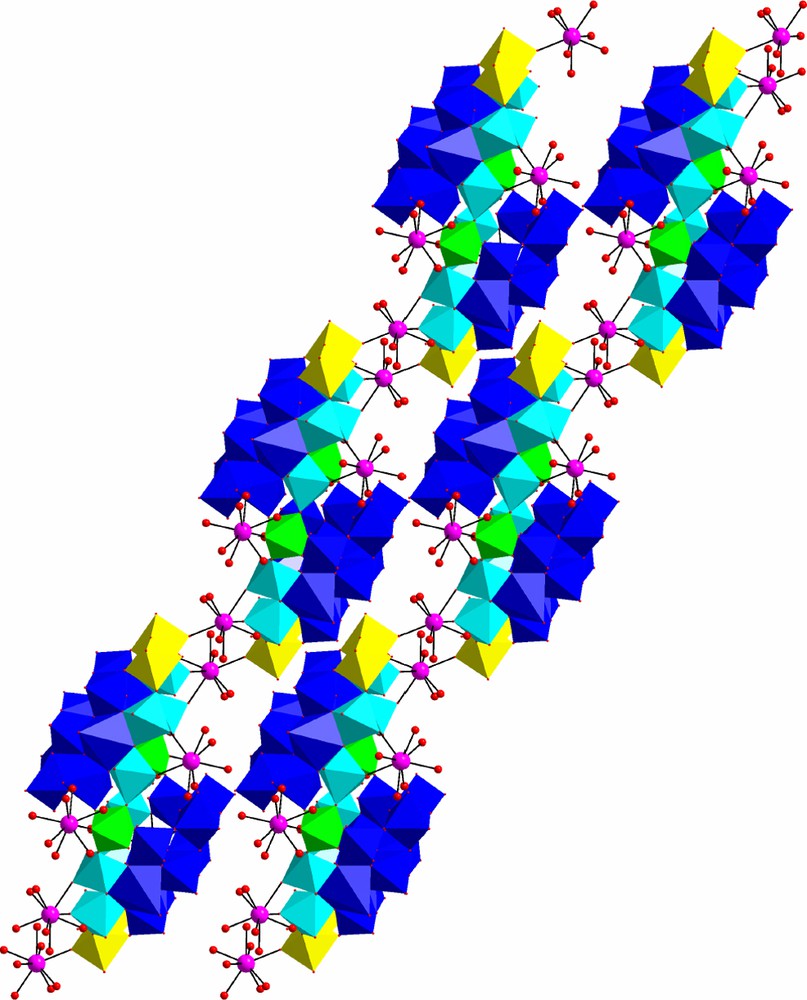
Two neighbor layers (view along b axis) in the crystal structure of 1. Crystallization water molecules are omitted for clarity. For color legend, see Fig. 1.
The structure of the title two-dimensional framework solid compound closely resembles that of recently published chain-like polymeric compounds [La2(MoO)2Mo36(NO)4O108(H2O)16]·68 H2O (2) and (H3O)12{[Mo2O5(H2O)2][Mo36(NO)4O108(H2O)16]}·44 H2O (3) [11,12]. Compound 2 was prepared by directly from [Mo36(NO)4O108(H2O)16]12– and La3+ in the presence of hydroxylammonium chloride [11]. Two electrophilic MoO3+groups in 2 are weakly coordinated by type A cis-MoO22+. Chain-building is through type A-MoO22+–La3+ (CN 9)–type C-MoO22+ interactions. In 3 the same [Mo36(NO)4O108(H2O)16]12– repeating units are connected by bridging {Mo2O4(μ-O)(H2O)2} groups coordinated to A-type MoO22+ groups only (type A-MoO22+–{Mo–O–Mo}–type A-MoO22+ connectivity) [12]. Again, as in 1, two neighboring A-type MoO22+ groups behave as bidentate ligands. The different connectivity types of the {Mo36(NO)4} unit in 1–3 indicate that the same cluster unit can be assembled in various ways and give products of different dimensionality if adopting different linkage centers.
The potential of terminal cis-MoO22+ groups in POM as ligands for building coordination polymers was recognized some time ago. The rare-earth ions are efficient linkers here, as shows recent preparation of 1D chain polymer [La(H2O)7Al(OH)6Mo6O18]·4 H2O [13], or (NH4)6[Gd2Mo36O112(H2O)22]·50 H2O [14].
The formation of a nitroso-POM, [Mo36(NO)4O108(H2O)16]12–, from the keplerate in the presence of Gd(III) nitrate is somewhat unexpected. We think that the Mo(NO)3+ forms in the slow reaction between NO3– and Mo(V), present in the keplerate as Mo2O42+ units. Traditional way to [Mo36(NO)4O108(H2O)16]12– is to reduce molybdate with hydroxylammonium [9–12]. Another type of keplerate degradation was observed in the presence of Ni2+, by a selective excision of Mo2O42+ moieties and their assembly into an ε-Kegging type structure [MoV12O30(µ2-OH)10H2{NiII(H2O)3}4], together with four attached Ni2+ ions [15].
4 Supplementary materials
The supplementary material has been sent to the Fachinformationzentrum Karlsruhe, Abt. PROKA, 76344 Eggenstein-Leopoldshafen, Germany, as supplementary material No. 391260 (CIF) and can be obtained by contacting the FIZ (e-mail: crysdata@fiz-karlsruhe.de or www: http://www.fiz-karlsruhe.de/fiz/products/icsdcsde.html).
Acknowledgments
This work was supported by the Russian Foundation for Basic Research, grant No. 02-03-32604 and INTAS, grant no. 01-2346. NVI thanks Haldor Topsoe A/S for a fellowship. MNS is thankful to the Russian Science Support Foundation for a grant.


