1 Introduction
Significant progress in research on nine-atom clusters of germanium has been made in recent years [1,2]. Typically, the clusters are obtained by dissolving alkali-metal/germanium precursors in ethylenediamine or liquid ammonia followed by crystallization with the aid of various sequestering agents for the alkali-metal countercations. The sequestering agents, typically crypts or crown ethers, are not necessary for the dissolution process. The precursors of stoichiometry A4Ge9 (A = Na, K, Rb) can be dissolved in ethylenediamine at room temperature without a sequestering additive. The latter is added later in order to crystallize a compound. The shape of the Ge9-clusters is that of a tricapped trigonal prism with elongated one, two or three prismatic edges, those parallel to the threefold axis of the prism, and the clusters can carry charges of 4–, 3– or 2–. We have already established that these differently charged clusters coexist in the ethylenediamine solutions [3]. Depending on the type of sequestering agent and its concentration, compounds with clusters of different charges can be crystallized from such solutions.
One of the most noticeable recent discovery is that these clusters can couple to form dimers, [4] trimers, [5] and even tetramers, [6] [Ge9–Ge9]6–, [Ge9 = Ge9 = Ge9]6–, and [Ge9 = Ge9 = Ge9 = Ge9]8–, respectively. It was found that this depends to a great extent on the degree of concentration of clusters in the solution and also on the type of the sequestering agent. This and the observed infinite chains of such clusters, (Ge92–)∞ in [K(18-crown-6)]2Ge9 and [K(2,2-diaza-18-crown-6)]KGe9·3en, [7,8] provide hopes for achieving controlled stepwise growth of even larger oligomers in the future. Furthermore, the fact that these clusters can carry different charges, i.e. 2–, 3–, or 4–, makes their redox chemistry interesting and offers possibilities for their use as building blocks in materials with extended structures.
Here we report on the synthesis and characterization of a third compound with infinite chains of (–Ge92––)∞, [Rb2(4,2,1,1-crypt)]Ge9·en (1). Furthermore, the sequestering agent used for capturing the alkali-metal cations, 2,1,1-crypt (4,7,13,18-tetraoxa-1,10-diazabicyclo[8.5.5]eicosane), undergoes a condensation reaction of dimerization and forms a new compound that can be named 4,10,13,19,25,29,33,38-octaoxa-1,7,16,22-tetraazatricyclo [20,8,5,5]tetracontane, or 4,2,1,1-crypt for short.
2 Experimental section
2.1 Synthesis
All manipulations and reactions were carried out under argon. A precursor of nominal composition Li2RbGe17 was synthesized from a stoichiometric mixture of the elements (Li, Acros, 99+%; Rb, Strem, 99+%; Ge, Acros, 99.999%). The mixture was heated at 900 °C for 2 days in a niobium tubular container sealed by arc welding at both ends and then jacketed by flame-sealing under vacuum in a fused-silica ampoule. According to X-ray powder diffraction (Cu Kα1 radiation, in a Guinier camera under vacuum) the product contains Rb4Ge9, Li7RbGe8, and a side product of NbGe2 from a reaction with the container. Approximately 30 mg of the precursor were placed in 1 ml of ethylenediamine (Aldrich, redistilled, 99.5+%, packaged under nitrogen) and formed brown solution. Large amount of precursor remained undissolved, most likely the insoluble NbGe2. 32 mg of 2,1,1-crypt (liq. 90%, Aldrich packaged in ampoules) was added and the resulting green–brown solution was layered with 3 ml THF (anhydrous, 99.9%, Aldrich, packaged under nitrogen) in a test tube. Red crystals of [Rb2(4,2,1,1-crypt)]Ge9·en (1), in substantial amount, grew on the walls and the bottom of the test tube and were collected 2 days later.
2.2 Structure determination
X-ray diffraction data of a single crystal of 1 were collected at 100 K on a Bruker APEX diffractometer with a CCD area detector (graphite-monochromated Mo Kα radiation, crystals protected with Parathone-N oil). The structure was solved by direct methods and refined on F2 using the SHELXTL V5.1 package (after absorption correction with SADABS). Details of the data collection and refinement are given in Table 1. Further information on the crystal structure investigation is available free upon request from the Cambridge Crystallographic Data Center (12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336 033; http://www.ccdc.cam.ac.uk/conts/retrieving.html; or deposit@ccdc.cam.uk) upon quoting depository number CCDC 248253.
Crystallographic data for [Rb2(4,2,1,1-crypt)]Ge9·en
| Fw | 1457.09 |
| Crystal color, size (mm) | Red, 0.19 × 0.10 × 0.04 |
| Space group, Z | P, 2 |
| a (Å) | 11.1927(5) |
| b (Å) | 15.0516(7) |
| c (Å) | 15.7847(7) |
| α (°) | 57.671(1) |
| β (°) | 83.594(1) |
| γ (°) | 80.217(1) |
| V (Å3) | 2420.8(2) |
| ρcalc (g cm–3) | 1.999 |
| μ (cm–1) | 7.549 |
| 2 θmax (°) | 50 |
| R1/wR2 (I ≥ 2σ1)a (%) | 4.72/9.74 |
| R1/wR2 (all data) (%) | 8.00/10.94 |
a R1 = Σ||Fo|–|Fc||/Σ|Fo|, wR2 = {[Σ[(Fo)2–(Fc)2]2]/[Σw(Fo2)2]}1/2 for Fo2 > 2 σ(Fo2), w = [σ2(Fo )2 + (0.0469 P)2]–1 where P = [(Fo)2 + 2 (Fc)2]/3.
3 Results and discussion
The new compound [Rb2(4,2,1,1-crypt)]Ge9·en crystallizes in the centric triclinic space group P-1. The asymmetric unit consists of one Ge9 deltahedral cluster, one complex dication [Rb2(4,2,1,1-crypt)]2+, and one ethylenediamine molecule. The cluster has the shape of a distorted tricapped trigonal prism with one elongated prismatic edge (one of the three edges parallel to the threefold axis). The edge elongation creates an open square-like face, and the cluster can be viewed also as a monocapped square antiprism. The clusters are linked to each other by two exo-bonds at the two atoms of the elongated edge (the shorter diagonal of the open square-like face) and form an infinite chain (Fig. 1). The clusters alternate ‘up’ and ‘down’ within the chain, exactly as observed before in [K(18-crown-6)]2Ge9 and [K(2,2-diaza-18-crown-6)]KGe9·3 en [7,8]. The intercluster bonds of 2.493 and 2.475 Å compare well with the corresponding bonds in the latter two compounds and correspond to a single Ge–Ge bond.
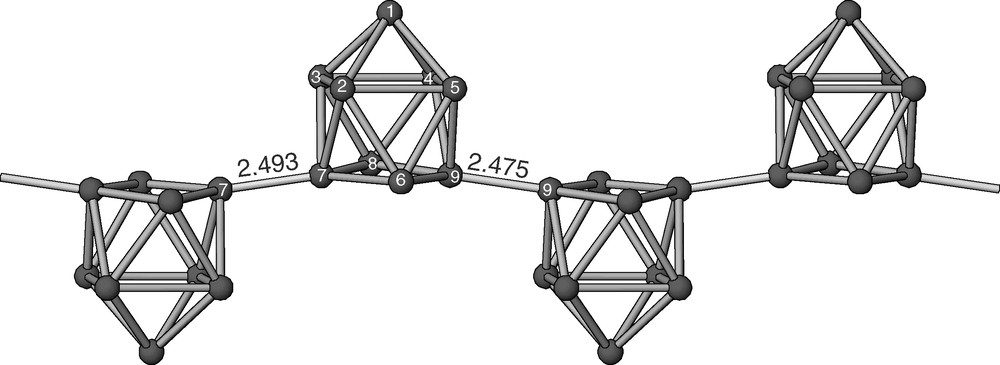
The infinite chain of clusters (–Ge92––)∞ in 1. The intercluster distances (shown) correspond to a Ge–Ge single bond distance. Intracluster distances (Å): 1–2 2.598, 1–3 2.577, 1–4 2.575, 1–5 2.581, 2–3 2.774, 2–5 2.724, 2–6 2.640, 2–7 2.642, 3–4 2.770, 3–7 2.620, 3–8 2.627, 4–5 2.805, 4–8 2.629, 4–9 2.627, 5–6 2.642, 5–9 2.635 6–7 2.548, 6–9 2.550, 7–8 2.554, 8–9 2.544 (standard deviations of 0.001 Å for all distances).
Perhaps the most interesting aspect of the new compound is the dimerization reaction of the sequestering agent 2,1,1-crypt that occurs during the synthesis. This involves the opening of a C–N bond in one of the two single-oxygen chains in each 2,1,1-crypt followed by restoration of the same type of bonds but with partners from different molecules. The resulting dimer has one large ring (Fig. 2) that contains 4 nitrogen, 6 oxygen, and 20 carbon atoms. Although somewhat wavy, the ring can be considered as defining a plane (Fig. 2b), just like the crown polyether does. Attached to pairs of nitrogen atoms in this ring are the two remaining single-oxygen links of the original 2,1,1-crypts. It is important to point out that the two linkages are on the same side of the plane defined by the large ring, and the dimer can occupy only a half of the coordination spheres of the sequestered rubidium cations. This is very similar to the way crown ethers coordinate to large cations, usually on one side of the cation. On the other hand, it is very different from cryptands, which wrap the whole cation and do not leave any opening in its coordination sphere. Therefore, a cation sequestered in cryptand is completely incapable for any interactions with the anionic part of the structure. In contrast, crown ethers as well as the dimer of 2,1,1-crypt in the present case leave the cations quite exposed for additional interactions and they coordinate to the chain (Fig. 3) and stabilize it. It can be argued that, perhaps, it is this coordination of cations that causes the formation of the chain itself. It should be noted that similarly the tetramer of [Ge9 = Ge9 = Ge9 = Ge9]8– is stabilized by eight rubidium cations sequestered by 18-crown-6 [6].

Two views of the new molecule named 4,2,1,1-crypt that is made of two 2,1,1-crypt molecules. A C–N bond is broken in each of the latter and two new intermolecular C–N bonds (open) are formed. The side view in (b) shows that the Rb+ cations have open coordination hemispheres for further interactions.
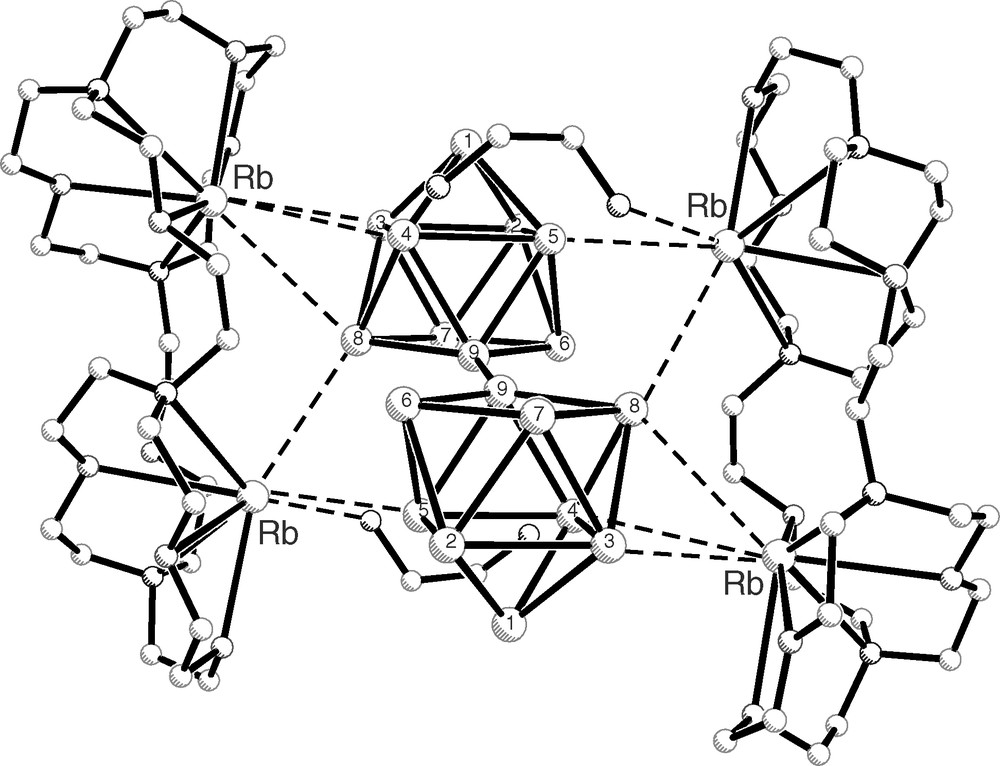
Shown are the interactions between the sequestered rubidium cations and the chain of clusters (the view is approximately along the chain of cluster). One of the cations interacts with a triangular face of one cluster while the second interacts with two atoms of two different clusters and a nitrogen atom from the solvent ethylenediamine captured in the structure.
The reaction of dimerization of the 2,1,1-crypt needs to be discussed in some more detail. First of all, it should be recognized that ethylenediamine solutions of alkali-metal containing main-group intermetallics are highly reducing and contain solvated free electrons, the ultimate reducing agent. Thus, the first step in the dimerization reaction is a molecule of 2,1,1-crypt taking an electron and breaking a C–N bond as a result of that. The extra electron will most likely reside on the more electronegative nitrogen and provide a two-bonded N– and a very reactive radical –CH2• end (Scheme 1). The process may be assisted by the lithium cations present in the solution which would insert inside the crypt and add more strain to the C–N bonds of the single-oxygen chains. An empty open molecule would coordinate directly a rubidium cation, while a molecule with lithium inside would replace the cation with rubidium due to the expected better complexation of the latter. The radius of Rb+ is larger than the cavity of a closed 2,1,1-crypt, and therefore, the cation would keep the molecule open (Scheme 1). It should be pointed out that 2,1,1-crypt is a strained molecule to start with, especially at the single-oxygen rings, one of which is the ring that opens in this case. Molecules of this type most likely open and close constantly at the C–N bonds when in solutions with free electrons even when without cations larger than the molecule's cavity. However, in the presence of large cations the molecule may not be able to close its original C–N bond and rather forms a bond to another molecule. How exactly this latter step is accomplished, i.e. the type and mechanism of the reaction, is not clear at all. One possibility is for the –CH2• radical to attack the nitrogen of another molecule and cause opening of a C–N bond in the latter with creation of the same radical end on the second molecule. Another possibility is for the nucleophilic N– of the first molecule to attack a carbon atom of the second molecule causing a bond opening and formation of another N– species. This process is also accompanied with insertion of another Rb+ in the second molecule. In both cases the last step must be recombination of N– and –CH2• with release of an electron.

One, perhaps, less important difference between compound 1 and the two other compounds with chains of germanium clusters is that while the color of the latter is reported as blue-green, the crystals of 1 are red. As it was already mentioned, both the inter- and intra-cluster distances in the three compounds are very similar, and the shapes of the clusters are almost identical. Therefore, the difference in color must be related to the cations captured in this unprecedented sequestering agent and their interactions with the chain. After all, it should be recognized that the chains do not exist in solution but rather assemble only in the solid product. Thus, the color of the solutions for all three compounds is the same, dark green to green-brown. In other words, they all contain the same or very similar species, most likely oligomers of three, four, or more clusters as we have shown before [5].
Acknowledgements
We thank the National Science Foundation (CHE-0446131) for the financial support.


