1 Introduction
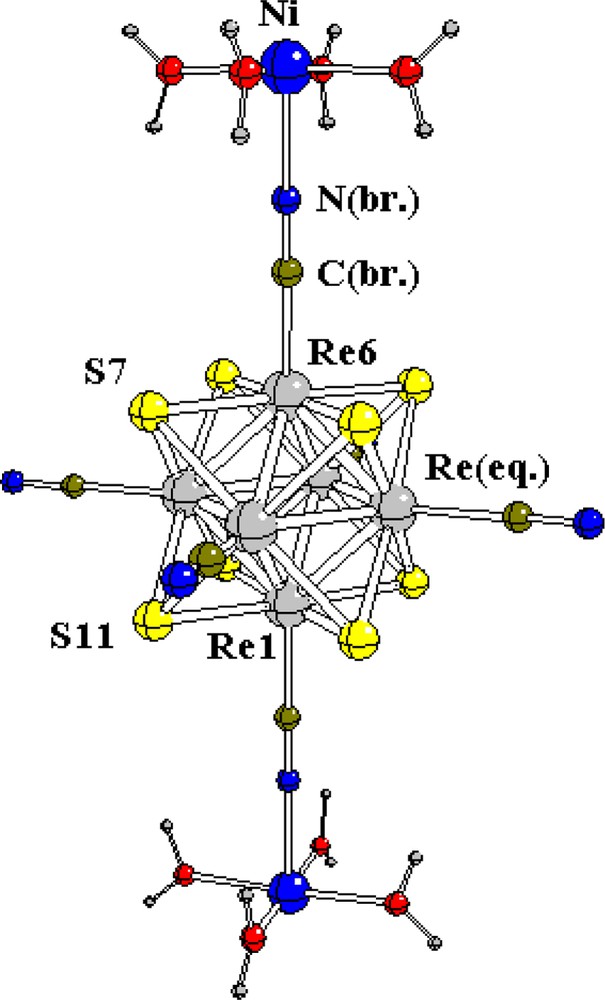
Octahedral chalcocyanide clusters [M6X8(CN)6]n– (M = Mo, Re, X = S, Se, Te) [1–4] are promising as a source of heterometallic polymers based on the ambivalent nature of cyanide ion, which allows formation of chains or 2D and 3D networks with intercluster links through transition metal M′ (M′ = Ni, Co, Mn, Fe, Cu) ions {–CN–M′(H2O)n–NC–} or through hydrogen bonds. Recently we investigated the influence of coordination of chromium carbonyl complexes to octahedral [Mo6Q8(CN)6]n– clusters on the structure of the Mo6 core [5]. It was shown that this interaction leads to considerable deformation of the metal octahedron. This effect was explained on the basis of the second order Jahn–Teller effect and/or the possible donor–acceptor property of the carbonyl and PR3 ligands. In this paper we describe the results of a quantum chemical study of the effect of interaction of the Re and Mo octahedral clusters with transition metal aquacomplexes. We present the results of quantum chemical calculations for the basic cluster [Re6S8(CN)6]4– (1) and for the corresponding bimetallic compounds: [Re6S8(CN)6–Ni(H2O)5]2– (2), {Re6S8(CN)6}{(Ni(H2O)5)2} (3) and [Mo6S8(CN)6–Ni(H2O)5]4– (4). The structure of (3), together with the atoms numbering scheme, is shown in Fig. 1.
Molecular structure of the [Re6S8(CN)6PH3]6– ionic pair.
2 Method of calculations
We used ab initio RHF and DFT (B3LYP functional) methods to investigate the electronic structure and to optimize geometry of the complexes under consideration. The calculations were performed with the Lanl2DZ basis set with the corresponding effective core potentials (ECP) [6]. The energies of some of the low-lying excited states of the clusters (1) and [Mo6S8(CN)6]6– (5) were calculated using the time-dependent DFT technique. All calculations were performed with the GAMESS [7] and GAUSSIAN03 [8] program packages.
Geometry optimization for all complexes was performed under no symmetry constraints. Results of geometry optimization are presented in Table 1. For [Re6S8(CN)6–Ni(H2O)5]2– the results of RHF calculations seem to be in better agreement with experimental data [4] than DFT calculations:
| Atomic pair: | Re–Re | Re–S | Re–C | Ni–N | Ni–O |
| Calculated interatomic distances (Å): | |||||
| B3LYP/Lanl2DZ | 2.630, 2.654 | 2.493, 2.516 | 2.044, 2.078 | 2.270 | 2.046 |
| RHF/Lanl2DZ | 2.616, 2.591 | 2.485, 2.504 | 2.101 | 2.069 | 1.990 |
| Experiment | 2.596, 2.602 | 2.391, 2.401 | 2.206, 2.12 | 2.04 | 2.14 |
Optimized bond lengths (in Å) for compounds 1, 2, 3, and 4 with the Lanl2DZ basis set
| Complex | (1) | (4) | (1) | (2) | (3) | (1) | (2) |
| Method | RHF | RHF | RHF | RHF | RHF | B3LYP | B3LYP |
| M6–M(eq) | 2.678 | 2.714 | 2.609 | 2.591 | 2.595 | 2.651 | 2.649 |
| M(eq)–M(eq) | 2.678 | 2.651, 3.118 | 2.616 | 2.610 | 2.651 | 2.630 | |
| M(1)–M(eq) | 2.678 | 2.704 | 2.603 | 2.595 | 2.651 | 2.654 | |
| M6–C(br.) | 2.257 | 2.146 | 2.056 | 2.060 | 2.044 | ||
| M(eq)–C | 2.257 | 2.177 | 2.116 | 2.101 | 2.080 | 2.096 | 2.078 |
| M1–C | 2.257 | 2.157 | 2.060 | 2.071 | |||
| S7–M6 | 2.526 | 2.506, 2.490 | 2.493 | 2.496 | 2.480 | 2.515 | 2.500 |
| S7–M(eq) | 2.526 | 2.529 | 2.504 | 2.470 | 2.524 | ||
| S11–M1 | 2.526 | 2.507 | 2.483 | 2.480 | 2.493 | ||
| S11–M(eq) | 2.526 | 2.571 | 2.485 | 2.470 | 2.516 | ||
| C(br.)–N | 1.146 | 1.163 | 1.167 | 1.173 | 1.150 | 1.195 | 1.197 |
| N–Ni | 2.167 | 2.069 | 2.110 | 2.270 | |||
| Ni–O | 1.970, 2.054 | 1.990 | 1.984 | 2.046 | |||
| ∠N–Ni–O | 90.9 | 87.2 | 88.8 | 82.0 |
3 Results
3.1 Molecular orbital schemes
Molecular orbital composition and energy levels schemes for clusters (1) and (5) have been reported earlier [9]. After formation of the ionic pairs the changes in the composition of the highest occupied molecular orbitals of the {Re6S8(CN)6} fragment are minor. The energies of the Ni ion orbitals are about 8.4 eV more negative than those of the Re–S orbitals. The electron density of the orbitals localized on the CN group is redistributed in favor of the equatorial ligands.
3.2 Geometry changes
The ion pairs of the rhenium cluster with [Ni(H2O)4]2+ can be regarded as the models of the real systems (Pr4N)2[{Ni(H2O)4}{Re6S8(CN)6}] (chain structure with the bridging Ni aquacomplex [4]) and of (Bu4N)2[{Ni(H2O)5}{Re6Se8(CN)6}] 2H2O [3]. In (2) and (3) the deformation of the Re6 core due to the addition of the [Ni(H2O)4]2+ ions is minor, the main change is a small (~0.05 Å) contraction of the apical Re(6)–C bond distances. This result is in good agreement with experimental data. Indeed it follows from the X-ray structure data [4] that there is really a distortion of the Re6 octahedron, but that the deviations from the mean values of the Re–Re distances are small (not exceeding 0.03 Å).
3.3 Electron-density redistribution
In the Re complexes with [Ni(H2O)4]2+, the charge transfer to the Ni complex is about 0.13 electron. In the Re6 octahedron the electron density redistribution leads to the decrease of the effective atomic charges on the equatorial atoms and to the increase of the negative charges on the Re atoms on the Re–Re–Ni axes (Table 2). Thus there is a shift of the electron density not only to the positively charged Ni fragment, but also in the opposite direction. At the same time in the sulfur moiety the electronic density moves only towards the Ni fragment.
Effective atomic charges (electron charge units) in the rhenium clusters (RHF/Lan2DZ calculations)
| [Re6S8(CN)6]4− | [{Ni(H2O)4}{Re6S8(CN)6}]2– | {Ni(H2O)4}2{Re6S8(CN)6} | |
| Re6 | –0.578 | –0.791 | |
| Re(eq.) | –0.570 | –0.412 | –0.464 |
| Re1 | –0.485 | –0.791 | |
| S | 0.334 | 0.282, 0.372 | 0.365 |
| CN(br.) | –0.541 | –0.654 | –0.593 |
| C(br.) | –0.151 | 0.097 | 0.076 |
| N(br.) | –0.390 | –0.751 | –0.669 |
| CN(eq.) | –0.541 | –0.519 | –0.498 |
| Ni | — | 1.323 | 1.330 |
In the model cluster {Ni(H2O)4}2{Re6S8(CN)6} the charge transfer (Δq) to each of the [Ni(H2O)4]2+ group is equal to 0.15 electron, that is almost equal to the Δq in the monosubstituted [{Ni(H2O)4}{Re6S8(CN)6}]2–. Thus the donation from the rhenium atoms to the [Ni(H2O)4]2+ groups is of local character. In general the electron density redistribution within the {Re6S8(CN)6} cluster can be described as its withdrawal from S atoms and equatorial Re atoms CN groups and its increase on the apical Re atoms and on the bridging CN groups. It is worth mentioning that there is a strong polarization within these CN groups (Table 2).
4 Discussion
In all cases, the geometry optimization of the [Re6S8(CN)6 (Ni(H2O)4)n]q systems leads to structures with a very small Re octahedron distortion. At the same time the calculations on the [Mo6S8(CN)6]q– adducts with the Cr(CO)4L fragments showed that inclusion of such groups results in noticeable changes in the basic framework of the Mo6 octahedron [5]. In this study such distortions were found also in the [Mo6S8(CN)6–Ni(H2O)5]4– ion pair. In [5], the large geometry changes in Mo clusters were partly attributed to the composition of the Cr(CO)4L fragments, which include such electronically active groups as CO and PR3 ligands. However, the calculations with the ‘normal’ complex Ni(H2O)42+ as a ligand showed that in this case the distortions of the Mo6 core are as large as those in the chromium adducts (Table 1). The specific calculations carried out for the [Re6S8(CN)6Cr(CO)5]4– adduct showed that the Re6 octahedron distortion is insignificant. This means that the specific donating or accepting properties of the ligands in the mononuclear complex are not responsible for distortions in the M6 metal clusters. Moreover, the calculated polarizabilities of the Re and Mo clusters are almost the same [9,10]. Therefore, it is reasonable to seek the explanation of such a different behavior of the Re and Mo compounds in their electronic structure, and first of all in the occupancy numbers of the valence orbitals of the parent clusters [Re6S8(CN)6]4– and [Mo6S8(CN)6]6–.
One well-known reason of structural distortion of molecular systems is the second order Jahn–Teller effect (or Jahn–Teller pseudoeffect) [11]. The necessary condition of such distortions is the existence of low lying excited states of irreducible representation Γf and normal vibrations of symmetry, the direct product of which,includes totally symmetric representation (Γi stands for the irreducible representation of the ground state, being A1g in the case of the clusters studied in this work). The values that characterize the coupling between the electronic structure and nuclear displacements, i.e. the measure of the influence of the nuclear displacements on the electron distribution, are the coupling matrix elements divided by the difference of the energies of the states Ψi and Ψf:(1)
The small energy difference (Ef − Ei) leads to pronounced distortion of the molecular structure.
Let us analyze the electronic structure of the rhenium and molybdenum complexes bearing in mind eq. (1). The number of the occupied orbitals in Mo and Re clusters is different according to the number of the metallic electrons in the M6 cores (20 in Mo6 and 24 in Re6). The most evident difference between the electronic structures of Re and Mo clusters is in the occupancy of the frontier orbitals: in [Re6S8(CN)6]4– the 10eg orbital is the highest occupied molecular orbital (HOMO), but in [Mo6S8(CN)6]6– this MO (10 eg) is the lowest vacant molecular orbital (LUMO). The analysis of the symmetry of some of the frontier orbitals shows that in both cases there are excited states with symmetry of the normal vibrations of octahedral systems (eg, t2g, t1u, t2u). So there are some active vibrations for [Re6S8(CN)6]4– as well as for [Mo6S8(CN)6]6–. However, according to our calculations, the differences (Ef − Ei) for the lowest spin singlet excited states are equal to 0.79 eV for [Mo6S8(CN)6]6– (transition t2u → eg) and 2.71 eV (transition eg → t1g) and 2.74 eV (excitation eg → a2g) for [Re6S8(CN)6]4–. Consequently, it is reasonable to expect that the changes in the geometry due to the second order Jahn–Teller effect are much more pronounced in the molybdenum species than in the rhenium clusters. This interesting problem deserves future investigations.
Acknowledgements
The work was supported by the INTAS grant 2000-00689. All calculations were performed in Petrodvoretz Telecommunication Center.


