1 Introduction
Transition metal telluride complexes bearing niobocene fragments are useful reagents for the synthesis of heterometallic telluride complexes and metal telluride clusters when reacting with binary transition metal carbonyls [1]. The main strategy in this chemistry uses a ligand cross-transfer (CO versus Te) from one metal center to the other. Finally, neutral or anionic telluride clusters are formed depending on the nature of the substituents at the metal centers [2–4].
Another strategy for the synthesis of metal telluride clusters employs the oxidative decarbonylation of metal carbonyls by polytelluride anions [5–9]. In this context we were interested to cleave the Te–Te bond in Cp′2Nb(η2-Te2)H (Cp′ = tBuC5H4) (1) [10] by reductive means. In a first attempt reaction of 1 with CH3Li gave a red–violet, very reactive product of still unknown nature [11]. This product reacts with Co2(CO)8 to give a complex mixture, from which some compounds derived from the cubic body-centered Co9Te6 polyhedron (Scheme 1a) were obtained [11].

a) Co2(CO)8. b) Co2(CO)8, after 18 h addition of excess CH3MeI.
In this work we report on the attempt to alkylate the ionic compounds in this mixture by subsequent addition of CH3I. As a result we found the surprising formation of the neutral cluster [Co11Te7(CO)10], associated with and probably stabilized by the salt [Cp′4Nb2(CH3Te)Te]I both in solution and in the solid state.
2 Results and discussion
Reaction of a dark orange THF solution of 1 with 2 equiv. of CH3Li at room temperature gave an immediate color change to redviolet. Subsequent reaction of the solution with 2 equiv. of Co2(CO)8 caused vigorous gas evolution. The resulting dark mixture was refluxed for 18 h and then treated with 3 equiv. of CH3I at room temperature. Chromatographic work-up gave traces of the redbrown neutral complex 2 along with a mixture of dark brown salts containing the [Co9Te6(CO)8]– anion and 5 (Scheme 1b). Complex 2 is identical in its spectroscopic data with known {Cp′2Nb(CO)}2[Co9Te6(CO)8], which forms in much better yield in the reaction described in Scheme 1a [11].
The mixture of dark products was separated by chromatography and elution with CH2Cl2 and acetone into 3 and dark brown 5. The salt 3 may be transferred by metathesis reaction with PPh4Cl into [PPh4][Co9Te6(CO)8] [12], whereas crystallization from THF gave a few needles of [Na(THF)6][Co9Te6(CO)8] (4). The composition of 4 was proven by comparison of X-ray crystallographic data with those of an authentic sample [11]. The existence of 4 may only be explained by the presence of impurities of Na in the employed CH3Li.
The identification of 5 is based on electrospray ionization mass spectrometry (ESI-MS) and X-ray crystallography, the found composition is confirmed by elemental analysis. The PI-ESI-MS (solvent CH3CN, Tcap = 150 °C) exhibits one peak with center of gravity at 941.0 mu which may be assigned to the dinuclear cation [Cp′4Nb2Te2CH3]+. The negative ion (NI) ESI-MS of 5 reveals several peaks with intensities between 2% and 11% centered at 1628.3, 1598.3, 1570.2 and 1542.2 mu apart from the base peak at m/z = 1382.2. The relatively weak peaks may be assigned to the respective loss of seven, eight, nine or 10 CO ligands from the intact cluster ion [Co11Te7(CO)10]–. The observation of the peak at 1542 mu means that even the naked Co11Te7 cage exhibits a certain stability in the gas phase. However, the base peak may be derived from [Co9Te6(CO)8]– by loss of five CO ligands. As there is no proof in the crystal structure for the cubic polyhedron one may assume a transformation of the pentagonal-prismatic Co11Te7 cluster into the cubic Co9Te6 framework under mass spectroscopic conditions. A related rearrangement of the cluster framework as a consequence of CO loss during mass spectroscopic investigations was investigated quantitatively for [Pt3Ru10C2(CO)32]2– [13].
The IR spectrum of 4 exhibits a very strong absorption at 1973 cm−1 along with absorptions at 1478 and 1385 cm−1, which is in agreement with the ν(C–O–C) frequencies of coordinated THF ligands [11]. The IR spectrum of 5 reveals a strong and relatively broad absorption for terminal CO ligands at 1941 cm−1. Additional absorptions in the region of 2960 and 1365 cm−1 may be assigned to the tBuC5H4 ligands of the niobocene fragments. The 1H NMR spectrum of 5 is not clear-cut because of the low proton concentration of the sample, caused by the high molar mass of 5 and its low solubility. Resonances at δ = 1.46 ppm (tBu) and between 4.33 and 6.30 (multiplets of aromatic hydrogens) resemble those of [Cp′4Nb2Te2CH3]I, a compound which forms on methylation of Cp′4Nb2Te2 [10].
The crystal structure of 5 consists of two molecular units in the triclinic cell and each molecular unit contains one [Co11Te7(CO)10] polyhedron, one [Cp′4Nb2Te2CH3]+ ion, two I– ions (50% disordered each) and one CH2Cl2 molecule (Fig. 1). The polyhedra are stacked in parallel columns with respect to the a axis. These columnar structures are embedded in between two layers formed by the cations and the inversion centers of these cations lie in the ac plane (Fig. 1). The inversion centers of the disordered iodide ions are in the ac plane, too, but shifted by b/2. As the unit cell contains cations and anions in a 1:1 ratio the charge of the [Co11Te7(CO)10] cluster must be zero.
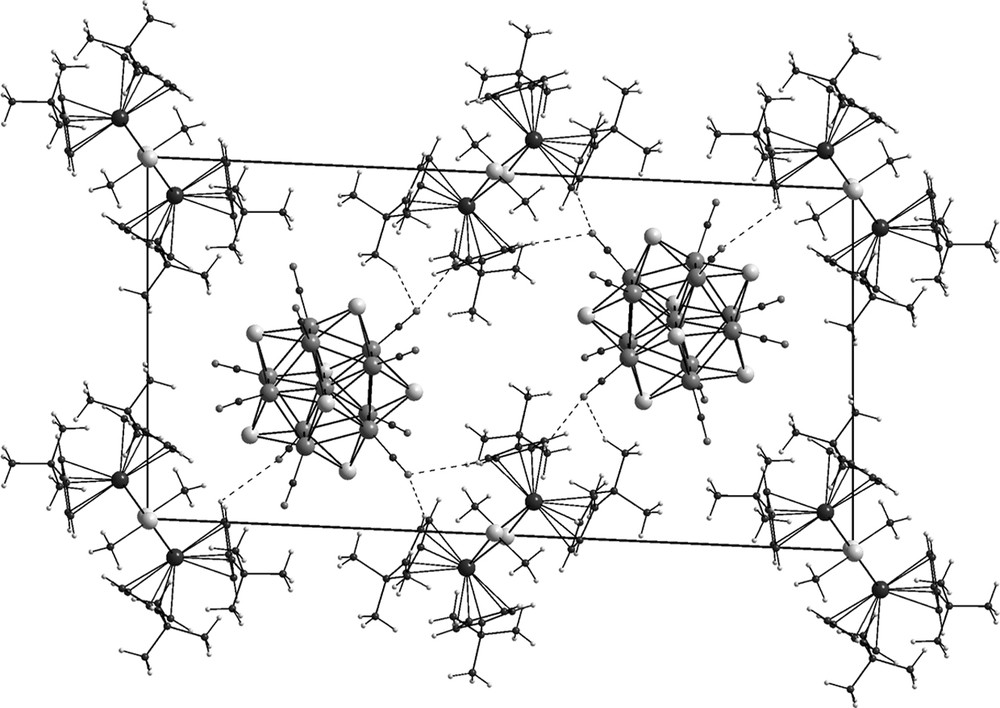
Projection of the crystal packing repeat of [Cp′4Nb2Te2CH3]I[Co11Te7(CO)10] (5) viewed down the a axis. O···H bonds between 2.50 and 2.85 Å are indicated by dotted lines. The disordered iodide ions and the solvent molecules (CH2Cl2) are not shown.
The structure of the [Cp′4Nb2Te2CH3]+ cation contains a planar rhombic four-membered Nb2Te2 ring with four attached Cp′ ligands, which are arranged in the achiral meso form (Fig. 2). This arrangement is similar to that in Cp′4Nb2Te2 [10,14] and [Cp′4Nb2(CH3Te)2]I2 [10] and similarities are also observed for the metric parameters (Table 1). Two methyl groups (C29) are fixed at the Te bridges in a trans position, but with an occupancy of 50% for each of the methyl groups. Accordingly, the molecule contains an inversion center. The composition is confirmed by the PI-ESI mass spectrum, which contains only the peak of the monomethylated species. It may be noted that mass spectrometry is an excellent tool to determine the methylation degree (one or two) of the parent dimer Cp′4Nb2Te2 after reaction with CH3I [10,14].
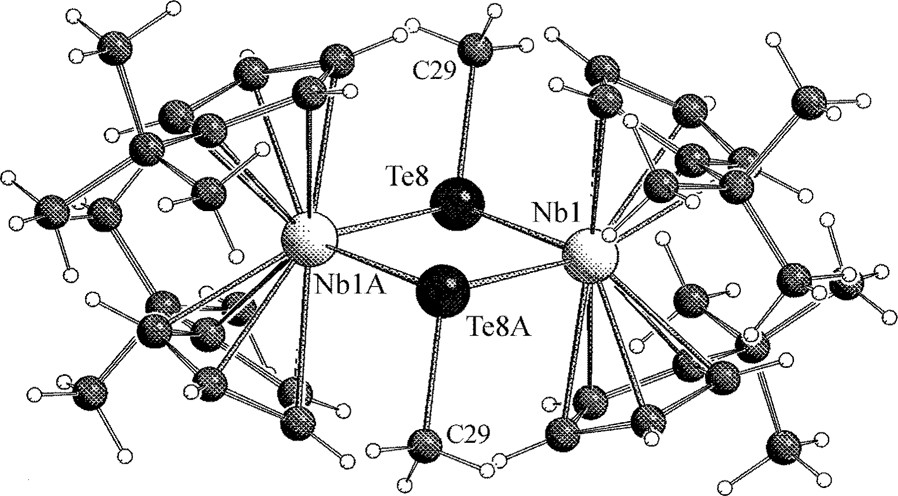
Structure of the [Cp′4Nb2Te2CH3]+ cation. The occupancy of each of the methyl groups C29 is 50%.
Selected distances (Å) and angles (°) for 5
| Te(1)–Co(11) | 2.6710(19) |
| Te(1)–Co(1) | 2.5834(18) |
| Te(1)–Co(2) | 2.5760(18) |
| Te(1)–Co(3) | 2.5805(17) |
| Te(1)–Co(4) | 2.5791(17) |
| Te(1)–Co(5) | 2.5943(17) |
| Te(2)–Co(6) | 2.5900(17) |
| Te(2)–Co(7) | 2.5736(17) |
| Te(2)–Co(8) | 2.5822(18) |
| Te(2)–Co(9) | 2.5839(18) |
| Te(2)–Co(10) | 2.5834(17) |
| Te(2)–Co(11) | 2.6795(19) |
| Te(3)–Co(1) | 2.5262(17) |
| Te(3)–Co(5) | 2.5196(18) |
| Te(3)–Co(6) | 2.5179(17) |
| Te(3)–Co(10) | 2.5239(18) |
| Te(4)–Co(1) | 2.5292(18) |
| Te(4)–Co(2) | 2.5226(18) |
| Te(4)–Co(6) | 2.5253(17) |
| Te(4)–Co(7) | 2.5225(16) |
| Te(5)–Co(2) | 2.5209(17) |
| Te(5)–Co(3) | 2.5238(18) |
| Te(5)–Co(7) | 2.5227(18) |
| Te(5)–Co(8) | 2.5191(17) |
| Te(6)–Co(3) | 2.5128(17) |
| Te(6)–Co(4) | 2.5236(17) |
| Te(6)–Co(8) | 2.5348(18) |
| Te(6)–Co(9) | 2.5301(18) |
| Te(7)–Co(4) | 2.5170(17) |
| Te(7)–Co(5) | 2.5269(18) |
| Te(7)–Co(9) | 2.5076(17) |
| Te(7)–Co(10) | 2.5129(19) |
| Co(1)–Co(2) | 2.590(2) |
| Co(1)–Co(5) | 2.588(2) |
| Co(1)–Co(6) | 2.631(2) |
| Co(1)–Co(11) | 2.569(2) |
| Co(2)–Co(3) | 2.583(2) |
| Co(2)–Co(7) | 2.614(2) |
| Co(2)–Co(11) | 2.556(2) |
| Co(3)–Co(4) | 2.588(2) |
| Co(3)–Co(8) | 2.623(2) |
| Co(3)–Co(11) | 2.563(2) |
| Co(4)–Co(5) | 2.568(2) |
| Co(4)–Co(9) | 2.650(2) |
| Co(4)–Co(11) | 2.558(2) |
| Co(5)–Co(10) | 2.639(2) |
| Co(5)–Co(11) | 2.559(2) |
| Co(6)–Co(7) | 2.586(2) |
| Co(6)–Co(10) | 2.575(2) |
| Co(6)–Co(11) | 2.562(2) |
| Co(7)–Co(8) | 2.583(2) |
| Co(8)–Co(9) | 2.568(2) |
| Co(8)–Co(11) | 2.558(2) |
| Co(9)–Co(10) | 2.583(2) |
| Co(9)–Co(11) | 2.559(2) |
| Co(10)–Co(11) | 2.557(2) |
| Nb(1)–Te(8) | 2.834(2) |
| Nb(1a)–Te(8) | 2.829(2) |
| Te(8)–C(29) | 2.15(3) |
| Co(2)–Te(1)–Co(3) | 60.1(1) |
| Co(2)–Te(1)–Co(4) | 108.1(1) |
| Co(2)–Te(1)–Co(5) | 107.9(1) |
| Co(1)–Te(1)–Co(3) | 108.5(1) |
| Co(1)–Te(1)–Co(4) | 108.0(1) |
| Co(1)–Te(1)–Co(5) | 59.9(1) |
| Co(4)–Te(1)–Co(5) | 59.5(1) |
| Co(1)–Te(1)–Co(2) | 60.3(1) |
| Co(3)–Te(1)–Co(4) | 60.2(1) |
| Co(3)–Te(1)–Co(5) | 107.8(1) |
| Co(1)–Te(3)–Co(10) | 94.1(1) |
| Co(1)–Te(3)–Co(5) | 61.7(1) |
| Co(5)–Te(3)–Co(6) | 94.0(1) |
| Co(1)–Te(3)–Co(6) | 62.9(1) |
| Co(6)–Te(3)–Co(10) | 61.4(1) |
| Co(5)–Te(3)–Co(10) | 63.1(1) |
| Te(1)–Co(1)–Te(4) | 118.7(1) |
| Te(3)–Co(1)–Te(4) | 109.3(1) |
| Co(2)–Co(1)–Co(5) | 107.7(1) |
| Co(2)–Co(1)–Co(6) | 89.6(1) |
| Co(5)–Co(1)–Co(6) | 89.6(1) |
| Co(1)–Co(11)–Co(5) | 60.6(1) |
| Co(1)–Co(11)–Co(7) | 92.1(1) |
| Co(1)–Co(11)–Co(9) | 149.6(1) |
| Te(1)–Co(11)–Te(2) | 179.32(7) |
| Nb(1)–Te(8)–Nb(1a) | 82.31(4) |
| Nb(1)–Te(8)–C(29) | 111.8(7) |
| Nb(1a)–Te(8)–C(29) | 115.4(7) |
The structure of the [Co11Te7(CO)10] cluster is in a close analogy to that of other clusters with the Co11Te7 framework [2,15,16]: a body-centered pentagonal prism of ten Co atoms is capped by five μ4-Te ligands and two μ5-Te ligands and each of the Co vertices bears a CO ligand (Fig. 3). A comparison of the bond parameters (Table 1) of the Co11Te7 skeleton of 5 with those of [Co11Te7(CO)5(PMe2Ph)5] [17] [Co11Te7(CO)10]– [2] and [Co11Te7(CO)10]2– [18] shows that deviations are at the limit of accuracy of the crystal structure determinations (Table 2).

Structure of the Co11Te7(CO)10 cluster.
Comparison of important distances (Å) of Co11Te7(CO)10 clusters in dependene of charge
| [Co11Te7(CO)10]0 [c] | Co11Te7(CO)5(PMe2Ph)5 [17] | [Co11Te7(CO)10]− [2] | [Co11Te7(CO)10]2− [18] | |
| m4-Te−Co | 2.508(2)–2.535(2) | 2.499(2)–2.545(2) | 2.498(3)–2.523(3) | 2.510(2)[b] |
| m5-Te−Co | 2.573(2)–2.594(2) | 2.550(2)–2.599(2) | 2.552(3)–2.581(3) | 2.569(2)[b] |
| Te−Cobc [a] | 2.671, 2.680 | 2.646(2), 2.673(2) | 2.631(3), 2.649(3) | 2.642(2), 2.647(2) |
| Co−Co | 2.568(2)–2.650(29 | 2.546(2)–2.655(2) | 2.522(4)–2.612(4) | 2.602(4)[b] |
| Co−Cobc [a] | 2.557(2)–2.569(2) | 2.532(2)–2.583(2) | 2.544(4)–2.572(3) | 2.558(3)[b] |
| Co−C [b] | 1.78(2) | 1.75(1) | 1.75(2) | 1.76(1) |
The uncharged [Co11Te7(CO)10] polyhedron possesses 147 valence electrons. Thus far it has been observed in electrochemical experiments after oxidation of solutions containing the [Co11Te7(CO)10]– anion (Scheme 2a) [2]. The violet salt [Cp′4Nb2Te2CH3]I can be prepared from Cp′4Nb2Te2 and 1 equiv. of CH3I (Scheme 2b). It is insoluble in THF, soluble in CH2Cl2 and does not migrate on silica gel. For these reasons one may discuss for the formation of 5 besides the possibility of a mere cocrystallization of both building blocks weak associative forces in solution, which prevents separation of one of the two compounds during work-up.

The organization of the distinct components in the unit cell may be promoted by attractive C−H···O contacts between hydrogen atoms of the tBu groups in the cation and oxygen atoms of terminal CO ligands of the cluster in the range between 2.50 and 2.89 Å (Fig. 1). The importance of hydrogen-bonding acceptor CO ligands for the crystal packing has already been discussed [15,16], and in our case it may be increased by electrostatic forces.
The role of large organometallic cations for the stabilization of anionic metal telluride clusters has already been mentioned [1]. The formation of the [Cp′4Nb2Te2CH3]+ cation cannot yet be explained and is therefore the subject of further investigations.
3 Experimental
3.1 General
All manipulations were carried out under nitrogen using Schlenk techniques and freshly dried and distilled solvents. Further information and the synthesis for Cp′2NbTe2H (1) are given in the reference [10].
3.2 Synthetic procedure
To the dark-orange solution of 1 (300 mg, 0.50 mmol) in 100 ml of THF was added 0.7 ml (1.1 mmol) of CH3Li in Et2O. The color changed immediately to red–violet and the mixture was stirred for 1 h at room temperature. Then Co2(CO)8 (342 mg, 1.0 mmol) was added and spontaneous gas evolution was observed. Finally, the dark mixture was refluxed for 18 h. After cooling 0.1 ml (1.6 mmol) of CH3I was added and the resulting mixture was stirred for an additional hour at room temperature.
After evaporation of the solvent the black residue was suspended in toluene. Filtration of the suspension gave a brown solution from which after chromatography on SiO2 16 mg (1.2%) of known 2 [11] were obtained. Extraction of the black residue was achieved with CH2Cl2 and then acetone to give two fractions which were purified by column chromatography (SiO2, activity II–III; column 20 × 3 cm). Thus, from the CH2Cl2-fraction a dark brown band was separated by elution with toluene/CH2Cl2 (1:1; v/v) containing 50 mg of a product of still unknown nature. Elution with CH2Cl2 gave a redbrown band containing 25–50 mg (6–12% yield with respect to Te in 1) of 5. Chromatography of the acetone-fraction gave after elution with toluene/acetone (1:1; v/v) two weak brown bands, which were discarded. With acetone a black band was eluted, containing 90 mg of 3. Dark needles were obtained by recrystallization from THF, which were confirmed by preliminary X-ray diffraction to contain [Na(THF)6][Co9Te6(CO)8] (4) [11]. Spectral data for 5: IR νCO (cm−1 in KBr) 1941(vs). Anal. Calc. for C47H55Co11INb2O10Te9·CH2Cl2 (2889.3) C(%); 19.38 H(%); 1.93. Found C; 19.73 H; 2.73. NI-ESI-MS (from CH2Cl2) 1628.3 ([Co11128Te7(CO)3]– = 1629, 10%), 1598.3 ([Co11Te7(CO)2]–, 11%), 1570.2 ([Co11Te7(CO)]–, 5%), 1542.2 ([Co11Te7]–, 2%), 1382.2 ([Co9Te6(CO)3]–, 100%); PI-ESI-MS (from CH2Cl2) 941.2 ([Cp′4Nb2Te2CH3]+, 100%).
3.3 Crystal structure determination of 5
Black plates of 5 were grown from CH2Cl2 at –24 °C. Data were collected at 173 K on a STOE imaging plate diffraction system using Mo Kα radiation (λ = 0.71073 Å). Crystallographic details are listed in Table 3. The structure was solved by direct methods and refined by full-matrix least-squares (SHELXL97 program) with all reflections. All nonhydrogen atoms were refined with anisotropic displacement parameters, the H atoms were calculated geometrically and a riding model was used during the refinement process. Due to the inversion centers at the midpoints of the C37H55Nb2Te2 cations, the methyl groups attached to the Te atoms show a positional disorder between the two possible orientations. The same is true for both iodine ions, which also are located next to inversion centers. After a refinement of the structure in the acentric space group P1, all splitted positions for these methyl groups and iodide ions appeared in the difference Fourier synthesis with exactly 1/2 of the expected electron number for these atoms. Therefore the description of the structure in the centric space group P is assumed to be correct.
Crystallographic data for compound 5
| 5 | |
| Formula | C37H55Nb2Te2, C10Co11O10Te7, CH2Cl2 |
| Molecular weight | 2974.19 |
| Crystal size (mm3) | 0.20 × 0.08 × 0.02 |
| Crystal system | Triclinic |
| a (Å) | 9.271(1) |
| b (Å) | 14.719(1) |
| c (Å) | 27.897(3) |
| α (°) | 85.32(1) |
| β (°) | 81.75(1) |
| γ (°) | 74.726(1) |
| V (Å3) | 3630.4(6) |
| Space group | P |
| Z | 2 |
| ρcalc (g cm–3) | 2.721 |
| Instrument | Stoe IPDS |
| Temperature (K) | 173 |
| μ (mm–1) | 6.832 |
| Absorption correction | Empirical |
| Transmission | 0.775/0.361 |
| Scan range | 2.01 < Θ < 25.78 |
| Total reflections | 12 829 |
| Observed reflections (I > 2.0σ(I)) | 7576 |
| Number of LS parameters | 766 |
| Residual density (e Å–3) | 5.786/–1.630 |
| R1 | 0.045 |
| wR2 | 0.096 |
Acknowledgements
We gratefully acknowledge financial support from the Deutsche Forschungsgemeinschaft.
Supplementary material
Crystallographic data for the structural analysis have been deposited with the Cambridge Crystallographic Data Center. The deposit number is CCDC 248624 for compound 5. Copies of this information may be obtained free of charge from the Director, Cambridge Crystallographic Data Center, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336 033; or e-mail: deposit@ccdc.cam.uk.


