1 Introduction
Surface-mediated solid phase reactions are of growing interest [1] because of their advantages of ease of set up, mild conditions, rapid reactions, selectivity, increased yields of the products and low cost compared with their homogeneous counterparts.
α-Amino phosphonates are an important class of compounds in modern pharmaceutical chemistry [2]. Peptido mimetics [3] made out of this class of compounds have shown promising pharmacological proprieties [4]. They also play an important role in hapten design for antibody generation [5] and enzyme inhibitors [6].
As a result, a variety of synthetic approaches have been developed for the synthesis of α-amino phosphonates. Of these methods, the nucleophilic addition of phosphates with imines [7], catalysed by an acid or a base is one of the most convenient methods. Lewis acids [8] are known to catalyse these reactions under mild conditions. Interesting applications of this type of reactions have been reported by Simoni et al. [9], who has developed a procedure for the synthesis of α-amino phosphonates using tetramethylguanidine as a catalyst. Recently, CdI2 [10] was also found to be effective for this transformation. However, CdI2 has been used in benzene and heating at 40–45 °C.
In this report, a new method for the synthesis of α-amino phosphonates on a solid surface is described. It was found that synthetic diphosphate Na2CaP2O7 under solvent-free conditions at room temperature is capable of the synthesis of α-amino phosphonates from imines and dialkyl phosphite under mild reaction conditions in few minutes (Scheme 1).

Synthesis of α-amino phosphonates in the presence the Na2CaP2O7 catalyst.
2 Experimental
2.1 Preparation of the catalyst and structural characteristics
The synthetic phosphate Na2CaP2O7 has been prepared by reaction between Na2CO3, CaCO3 and NH4H2PO4 (Scheme 2) as described previously [11]. The final product is identified by X-ray powder diffraction using a Siemens D-500 diffractometer (Cu Kα radiation 1.5406 Å; Space group: triclinic P
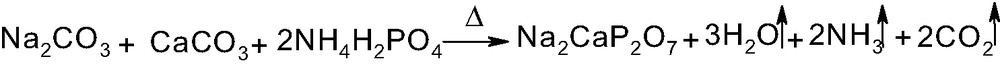
The structure is characterised by the presence of tunnels with similar dimension along the three directions [100], [010] and [001]. Fig. 1 gives a projection view of the structure along the crystallographic plane (100) [12]. Two kind of tunnels are present and both running along the [100] direction. They are built up from P2O7 groups and CaO6 octahedra. Their dimension could be estimated by the respective distances (d1 = 4.16 Å, d2 = 6.90 Å) and (d3 = 4.04 Å, d4 = 5.93 Å). This space is not totally free because it is reduced by the presence of sodium cations which exhibit two kind of crystallographic sites (Fig. 1). The size distribution of the powder particles was determined. The result shows that all the sizes were statistically distributed below 50 μm. The surface area of calcined Na2CaP2O7 was determined by the BET method from the adsorption–desorption isotherm of nitrogen at its boiling temperature, using a conventional volumetric apparatus and was equal to 2.4 m2 g–1.
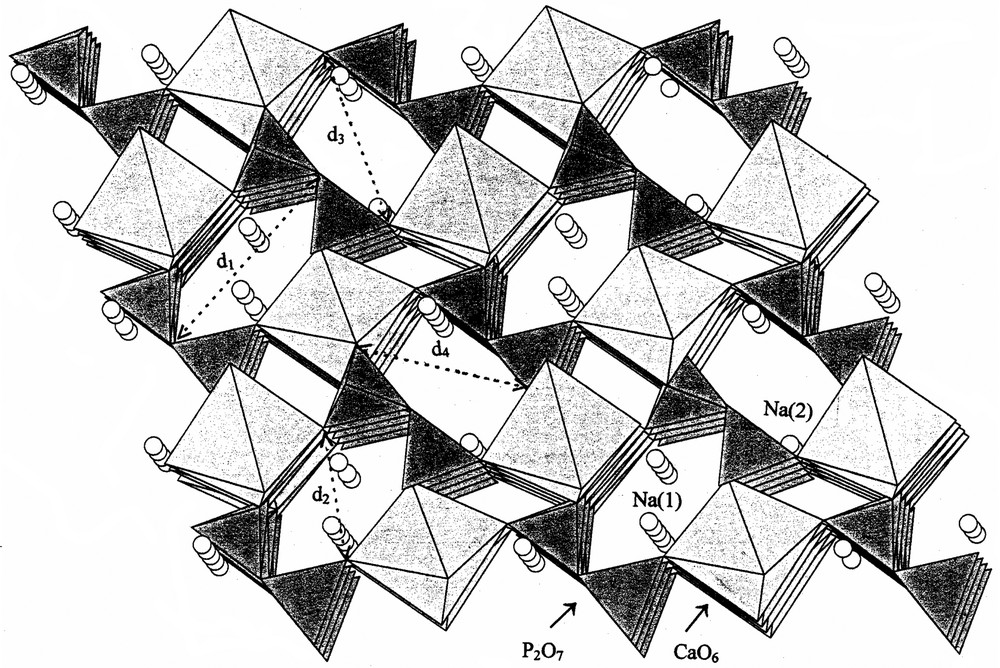
Projection view of Na2CaP2O7 on the (100) plane (d1 = 4.16 Å, d2 = 6.90 Å) and (d3 = 4.04 Å, d4 = 5.93 Å).
2.2 Preparation and characterisation of imine 1a–j
The imine 1a–j were easily prepared by simple chemistry starting from the commercially available amines and aromatic aldehydes under standard conditions [13] (see Scheme 3 and Table 1).
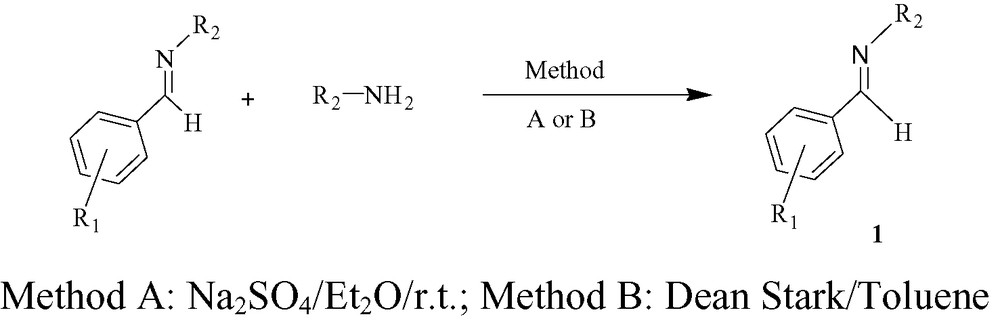
Preparation of imine 1a–j by two methods.
Synthesis of imine 1a–j by the amines and aromatics aldehydes under standard conditions
2.3 Materials and methods
1H and 13C NMR spectra were recorded at 400 and 100 MHz, respectively, on a Bruker DRX-400 spectrometer in CDCl3, using CDCl3 as internal standard. The chemical shifts (δ) are expressed in ppm relative to CDCl3 and coupling constant (J) in Hertz. IR spectra were obtained on a FTIR (ATI Mattson-Genesis Series) and reported in wave numbers (cm−1). Surface area and pore size analysis were carried out at 77 K on a Micromeritics ASAP2010 instrument using nitrogen as adsorbent. X-ray diffraction patterns of the catalysts were obtained on a Philips 1710 diffractometer using Cu–Kα radiation. Melting points were determined with a “Thomas Hoover” melting (capillary method) apparatus and are uncorrected. Flash column chromatography was performed using Merck silica gel 60 (230–400 mesh ASTM).
2.4 Typical experimental procedure
To a mixture of diphosphate catalyst 0.3 g and the imine 1 (1 mmol) was added dialkyl phosphite 2 (1.1 mmol). The mixture was stirred under solvent-free conditions at room temperature. The reaction progress with monitored by TLC (thin layer chromatography). The reaction mixture was diluted with dichloromethane, filtered out and the catalyst was washed with dichloromethane. After concentration of the filtrate under reduced pressure, the crude product was purified by silica gel column chromatography using n-hexane/ethyl acetate (80:20) as eluent. The product structure was analysed by 1H, 13C NMR melting points and IR spectrometry.
3 Result and discussion
To examine the catalytic activities of synthetic diphosphate Na2CaP2O7 [14] under solvent-free conditions at room temperature, we carried out the reaction of N-phenylbenzaldimine (R1 = H, R2 = Ph, Scheme 1) and diethyl phosphite, in presence of various amount of catalyst. After 15 min of reaction the yields of product 3a are summarised in Fig. 2. The best result was obtained with 0.3 g of Na2CaP2O7.
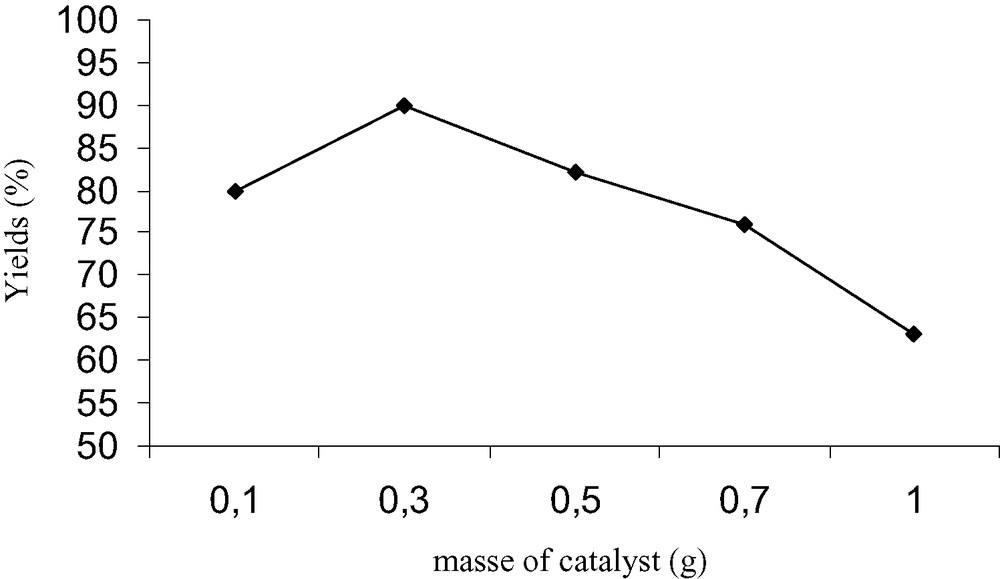
Influence of amount of Na2CaP2O7 in the synthesis of α-amino phophonate 3a, time of reaction 15 min.
In order to determine the scope and limitations associated with this catalyst, the optimum conditions for the reaction of N-phenylbenzaldimine (R1 = H, R2 = Ph, Scheme 1) and diethyl phosphite were applied to other substrate (Table 2).
Synthesis of α-amino phosphonates 3 in the presence the Na2CaP2O7 under solvent-free conditions at room temperature
As shown in Table 2, the imines 1 react with dialkyl phosphites 2 in the presence of synthetic phosphate Na2CaP2O7 to afford the desired products 3 in good to excellent yields in few minute (10–15 min).
The imine, possessing a phenyl substituent (R1 = H, R2 = Ph, Scheme 1) reacted effectively with diethyl phosphite to afford the α-amino phophonate 3a in excellent yields. Aromatic bearing an electron-donating substituent (R1 = p-MeO, p-Me) increase the reaction rate. Aromatic bearing an electron-withdrawing substituent (R1 = p-Cl, p-NO2) decrease the reaction rate. Steric hindrance at the ortho position of the N-aromatic ring seems to disfavour both kinetic and yield of the reaction (entries 3i/3a and 3j/3c).
Thus, we estimate that the surface of Na2CaP2O7 presents certainly multicatalytic active sites. The basic sites (oxygens of P2O7 group and CaO6 octahedra) abstract the proton from the phosphite. The acidic sites (phosphorus of P2O7 group, Na+ and Ca2+ cations) coordinate with the nitrogen of imine and facilitate the nucleophilic addition of dialkyl phosphite. Consequently, the C–P bond formation is facilitated and the final α-amino phosphonate is obtained by transfer of a proton.
We next investigated the stability of the catalyst in order to recycle it. The used and recovered Na2CaP2O7 has been shown to be reusable after drying at 150 °C under vacuum, and more efficiently after washing with acetone followed by calcinations at 600 °C (Fig. 3). In the last case, the catalyst can be recovered and reused at least five times without appreciable loss of activity.
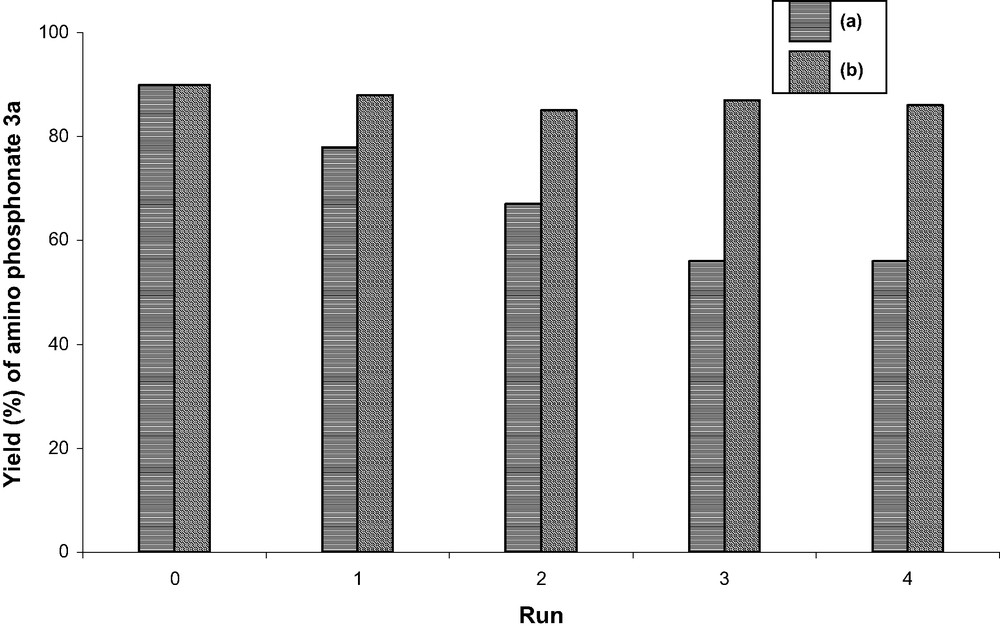
Recycling of the Na2CaP2O7 catalyst in the synthesis of 3a: (a) catalyst recoverable dried at 150 °C for 1 h; (b) catalyst recoverable, washed with acetone, dried and calcined at 600 °C for 30–60 min.
4 Conclusion
We have developed a novel and effective route to α-amino phophonate derivatives 3 by the addition reaction of the dialkyl phosphites 2 to imines 1 using the synthetic diphosphate Na2CaP2O7. This catalyst brings advantages such as high catalytic activity and selectivity under mild conditions avoiding toxic solvents and easy separation of the catalyst by simple filtration.


