1 Introduction
Preparation of cis and/or trans decalols by heterogeneous hydrogenation of the corresponding naphthols over supported metals as catalysts could be a short and challenging synthetic route if highly stereoselective. Hydrogenation of 1-naphthol and 2-naphthol have been studied at the beginning of the century over nickel [1–3], reduced copper [4], platinum [6,7] or rhodium [8] and regained but few attention in recent years [9] but ruthenium has not yet been used. Moreover, 1H NMR has never been used to determine the isomers' structure.
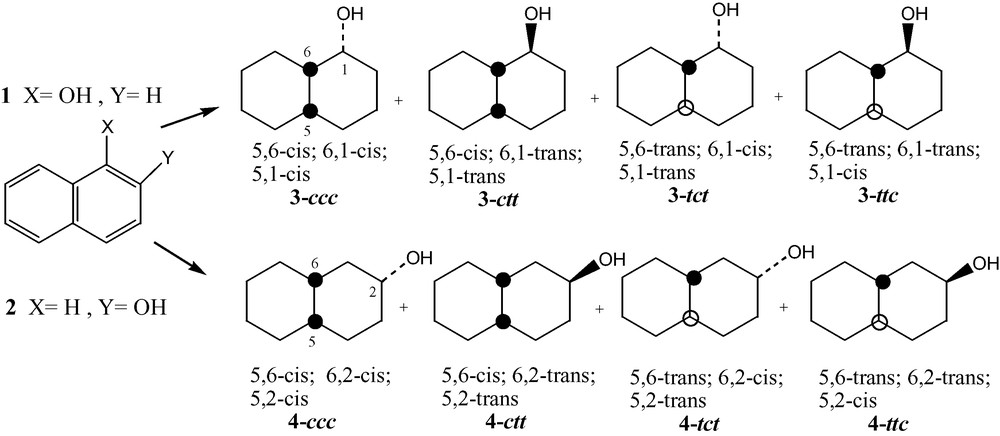
We present here a preliminary study of Ru/Al2O3 heterogeneous hydrogenation of 1-naphthol and 2-naphthol (1, 2) to obtain decalols 3 and 4, Fig. 1, as well as a simple, rapid and basic 1H NMR method which allows simultaneous determination of isomers' ratio and three-dimension structure of the four possible diastereomers formed.
2 Results and discussion
2.1 Hydrogenation of 1-naphthol
The results of hydrogenation of 1-naphthol are gathered in Table 1. Partially hydrogenated naphthol (5) was the major product obtained in THF, moreover, large amount of 5 was obtained in EtOH after 1 h but none after 4 h, suggesting that 5 is an intermediate in these hydrogenations. Compound 5 has also been observed and proposed as intermediate by Musser et al. [5] as well as Meyers et al. [8] with nickel, copper and rhodium catalysts.
Hydrogenation of 1-naphtol 1
| Starting material | Solvent | Temperature (°C) | React times (h) | 3-ccc/3-ctt/3-tct/3-ttc | 5% | cis/trans-decalin syst | Yield % in 3 |
| 1-Naphthol | EtOH | 40 | 1 | 37/14/0/1 | 48 | 98/2 | 52 |
| 1-Naphthol | EtOH | 40 | 2 | 238/14/2/1 | 45 | 95/5 | 55 |
| 1-Naphthol | EtOH | 40 | 4 | 74/26/0/0 | 0 | 100/0 | 100 |
| 1-Naphthol | THF | 40 | 4 | 0/0/0/5 | 75 | – | 5* |
It is interesting to note that the cis-decalin system is obtained exclusively (Table 1, lines 1 and 2) although mixtures of cis and trans-decalin systems are usually observed in the literature upon hydrogenation of 1-naphthol over other metals [5,8] or upon hydrogenation of naphthalene over Adams platinum oxide catalyst [7].
2.2 Hydrogenation of 2-naphthol
The results of hydrogenation of 2-naphtol are gathered in Table 2.
Hydrogenation of 2-naphtol 2
| Starting material | Solvent | Temperature (°C) | React times (h) | 4-ccc/4-ctt/4-ttc/4-tct | cis/trans-decalin syst. | Yield % |
| 2-Naphthol | EtOH | 40 | 4.5 | 38/0/0/62 | 38/62 | 100 |
| 2-Naphthol | THF | 40 | 5 | 48/0/5/47 | 48/52 | 100 |
| 2-Naphthol | Hexane | 40 | o.n.* | 34/0/0/66 | 34/66 | 100 |
Partially hydrogenated napthol is not observed in this case even in THF but mixtures of cis and trans-decalin systems are always obtained.
2.3 Structure assignment of 1- and 2-decalols
Assignment of the various isomers is a key step, therefore, a simple method based on very basic NMR is presented bellow. This method allows simultaneous determination of the three-dimensional structures and of the ratios between the isomers. It is worth noting that, although in this work most of the isomers have been isolated (chorotography over silica gel, using Et2O/hexane as eluent) for microanalysis and checking of their structure, the method allows the use of crude product of reactions.
Determination of the structure of the diastereomers of compounds 3 and 4 was done using the patterns of the methine proton on the carbon bearing the OH group and relative values of the 3J coupling constants observed. 1H NMR spectra of diastereomers mixtures and of isolated diastereomers have been used/compared and, of course, ring inversion equilibrium have been taken into account in the case of the non-rigid cis-decalols (3-c and 4-c), Schemes 1 and 2, as the values observed for the 3J coupling constants are the weighted average of the values of each conformers.


2.3.1 1-Decalols: predictions
In isomer 3-ccc, conformation K1-(OH equatorial) will be largely preferred over K2-(OH axial) because of syn-repulsion OH/CH2 in K2 [10] and one can thus expect for H1 a double triplet with one large coupling constant (3Jtrans) and two small (3Jgauche).
Conformation K1-(OH equatorial) will also be preferred in isomer 3-ctt but conformation K2-(OH axial) will not be so disfavored in this case because of no OH/CH2 syn-repulsion and one can expect for H1 a triplet of doublet with two averaged coupling constants (3Jtrans in K2 and 3Jgauche in K1) and one small (3Jgauche in both conformers), Scheme 1.
In the rigid trans-decalin system (3-t) the OH occupies a well-defined axial (3-tct) or equatorial (3-ttc) position providing for H1 a quadruplet (three small 3Jgauche) in 3-tct and/or a triplet of doublet (with two large 3Jtrans and one small 3Jgauche) in 3-ttc.
2.3.2 1-Decalols: observed
Four signals are observed and comparison of the predicted patterns with the observed ones then provides assignment of the four isomers of 1-decalols:
H1: 3.2 ppm; 3J = 10.5, 9.5, 4.5 Hz (two large and one small = DDd) ≡ 3-ttc.
3.68 ppm; 3J = 11, 4.5, 4.5 Hz (one large and two small = Dt) ≡ 3-ccc.
3.76 ppm; 3J = 3, 3, 3 Hz (three small = q) ≡ 3-tct.
3.83 ppm; 3J = 7.5, 7.5, 4 Hz (two averaged and one small = td) ≡ 3-ctt.
The results are also gathered in Table 3.
1H NMR de H1 et H2 (δ, coupling constant, pattern) predicted and observed for all possible diastereomers of 1-decalols (3) and 2-decalols (4)
| Start | 3-ccc | 3-ctt | 3-tct | 3-ttc | |
| 1 | Predicted | ||||
| Patterna | Dt | td | q | Td | |
| δ (ppm)b | 3.68 | 3.83 | 3.76 | 3.2 | |
| Patterna | Dt | td | q | DDd | |
| 3J (Hz) | 11; 4.5; 4.5 | 7.5; 7.5; 4 | 3; 3; 3 | 10.5; 9.5; 4.5 | |
| 4-ccc | 4-ctt | 4-tct | 4-ttc | ||
| 2 | Predicted | Tt | tt | Tt | pentuplet |
| Patterna | |||||
| δ (ppm)b | 3.82 | c | 3.58 | 4.1 | |
| Patterna | Tt broad | Tt | pent | ||
| 3J (Hz) | 9.5; 9.5; 4.5; 4.5 | 10; 10; 5; 5 | 3; 3; 3; 3 |
a Capitals correspond to large values of coupling constant (~10 Hz); small letters correspond to small values of coupling constant (1–8 Hz).
b 1H NMR in CDCl3/TMS.
c Not observed.
2.3.3 2-Decalols: predictions
The same analysis holds although H2 is now coupled with four vicinal protons instead of three, Scheme 2.
In isomer 4-ccc, conformation K1-(OH equatorial) will be largely preferred over K2-(OH axial) because of syn-repulsion OH/CH2 in K2 [10] and one can thus expect for H2 a triplet of triplet with two large coupling constant (3Jtrans) and two small (3Jgauche).
Conformation K1-(OH equatorial) will also be preferred in isomer 4-ctt but conformation K2-(OH axial) will not be so disfavored in this case because of no OH/CH2 syn-repulsion and one can expect for H2 a triplet of triplet with two averaged coupling constants (3Jtrans in K1 and 3Jgauche in K2) and two small (3Jgauche in both conformers), Scheme 2.
In the rigid trans-decalin system (4-t) the OH occupies a well-defined equatorial (4-tct) or axial (4-ttc) position providing for H2 a pentuplet (4 small 3Jgauche) in 4-ttc and a triplet of triplet (with two large 3Jtrans and two small 3Jgauche) in 4-tct.
2.3.4 2-Decalols: observed
In this case one isomer is not observed and two of them should exhibit similar patterns (both Tt), therefore, the only unambiguous assignment is the pentuplet (4.1 ppm) to isomer 4-ttc (H2 equatorial and not inverting trans-decalin system). Because the values of the large coupling constants are 10 and 9.5 Hz, it is reasonable to postulate that the two other signals are not due to 4-ctt for which one would expect an averaged value of about 7.5 Hz for the large 3J (see above). Then, both signals being Tt, the assignment was based on the width of the lines, and therefore, the Tt with narrow lines was assigned to the trans-decalin system (no inversion, no dynamic process) that is to 4-tct.
H2: 3.58 ppm; 3J = 10, 10, 5, 5 Hz narrow lines (two large and two small = Tt) ≡ 4-tct.
3.82 ppm; 3J = 9.5, 9.5, 4.5, 4.5 Hz broad lines (two large and two small = Tt) ≡ 4-ccc.
4.1 ppm; 3J = 3, 3, 3, 3 Hz (four small = pentuplet) ≡ 4-ttc.
The results are also gathered in Table 3.
3 Conclusion
Catalytic heterogeneous hydrogenation of 1-naphthol and 2-naphthol over Ru/Al2O3 has shown to work in quantitative yields under 20 bar of H2 and at 40 °C. It was shown that hydrogenation of 1-naphtol provides almost exclusively (100% and 98%, Table 1, lines 1, 2) the cis-decalols having the OH group either equatorial or axial while hydrogenation of 2-naphtol provides mixtures of cis and trans decalols having both the OH group equatorial in the ratio 1/1–1/2 (Table 2).
A rapid method based on simple 1H NMR (direct determination of values and numbers of the 3J involved in the pattern of the most deshielded proton-signal), which allows to identify and assign the diastereomers of 1-decalols and 2-decalols is described.
4 Experimental
1H (300 MHz) and 13C (75.4 MHz) NMR spectra were recorded on a Bruker AC 300 spectrometer with CDCl3 as solvent. Chemical shifts (δ) are given in ppm downfield from TMS as an internal standard. TLC, were performed on Merck's glass plates with silica gel 60 F254. Silica gel Si 60 (40–60 μm) from Merck was used for the chromatographic purifications. Naphthols were purchased from Aldrich and used without further purification.
4.1 General procedure for hydrogenation
A solution of the desired naphthol (0.5 mmol, 1 equiv.) in 5 ml of solvent with 0.03 equiv. of the catalyst (Ru/Al2O3-9001 from Engelhard) was stirred for the desired time in an autoclave under 20 bar of H2 (at 40 °C). The autoclave was equipped with a glass-socket and remaining air has been rapidly eliminated through two successive manipulations: vacuum-H2 admission. The mixture was then filtrated to eliminate the catalyst, which was rinsed with solvent and recovered. The joined organic phases were then evaporated under vacuum and the crude products, were analyzed by NMR, prior to purification. All the compounds were known compounds, which had correct 13C NMR and analysis within accepted errors.
Acknowledgments
We are grateful to the French ‘Ministère des Affaires étrangères’ for a grant to B.A. (D. No. 20003994).