1 Introduction
Ionic liquids have received widespread attention as mediums for a variety of reactions because of their properties such as very low or practically no vapor pressure, remarkable solubility behavior and the possibility of varying their structure to manipulate parameters like density, solubility and reusable ‘green’ solvent for chemical reactions [1–3]. Ionic liquids (ILs) or room-temperature molten salts have attracted much attention from chemists in recent years [4–7]. Representing a new class of non-molecular ionic compounds, ionic liquids were extensively investigated as reaction media (solvents) [8], molecular probes and ionic channel blockers [9], phase-transfer catalysts [10] and IL crystal applications [11–13].
Multi-component reactions (MCRs) have attracted considerable attention since an increasing number of organic chemical compounds are formed by multi-component reactions (MCRs) that convert more than two educts directly into their products by one-pot reactions. Further, they are performed without need to isolate any intermediate during their processes; this reduces time and saves both energy and raw material. They have merits over two-component reactions in several aspects including the simplicity of a one-pot procedure, possible structural variations and building up complex molecules [14]. One of these MCRs is the preparation of amidoalkyl naphthols. It is worthy to note that by amide hydrolysis reaction, 1-amidomethyl-2-naphthols can be converted to important biologically active 1-aminomethyl-2-naphthol derivatives. The hypotensive and bradycardiac effects of these compounds have been reported [15]. The preparation of amidoalkyl naphthols can be carried out by multi-component condensation of aryl aldehydes, 2-naphthol and acetonitrile or amide in the presence of Lewis or Brønsted acid catalysts such as HClO4–SiO2 [16], heteropolyacids [17,18], montmorillonite K10 clay [19], P2O5 [20], sulfamic acid [21], Brønsted acidic ionic liquid [22], cyanuric chloride [23], Sodium hydrogen sulfate [24], Ce(SO4)2 [25] and Sr(OTf)2 [26]. However, some of these methods are associated with prolonged reaction time, toxicity and low yields of desired product. Further, when a solid aldehyde or high amounts of catalyst is used, an organic solvent such as dichloroethane is needed [21].
In this article, there is growing interest in development of clean processes involving green catalysts. In recent years, ionic liquids have attracted much attention as a new class of green solvents and catalyst [27]. Thus, in continuation of our previous works on the application of reusable acidic ionic liquid in organic synthesis we decided to investigate the synthesis of amidoalkyl naphthol derivatives in the presence of 2-methylpyridinium trifluoromethanesulfonate ([2-MPyH]OTf) [28] as a green and highly efficient catalyst.
2 Results and discussion
In the present study, we describe a simple and green procedure for the preparation of amidoalkyl naphthol derivatives (4) via a three-component condensation reaction between aryl aldehydes (1), 2-naphthol (2) and amide (3) in the presence of [2-MPyH]OTf as a heterogeneous catalyst under thermal solvent-free conditions (Scheme 1).
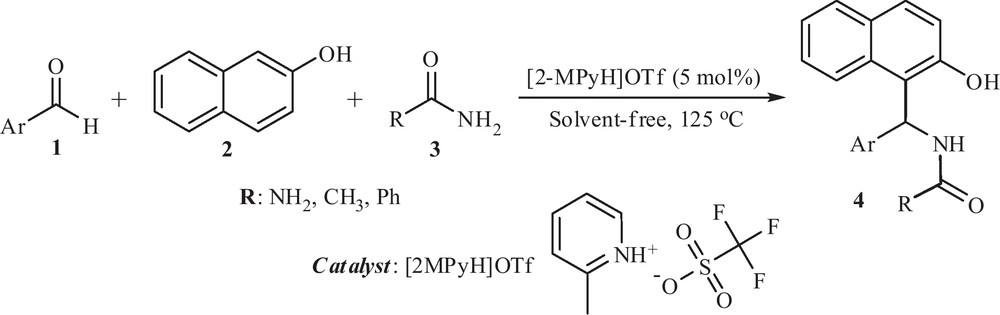
A simple green procedure for the synthesis of amidoalkyl naphthol using [2-MPyH]OTf.
To study the generality of this process, several examples illustrating this method for the synthesis those amidoalkyl naphthol derivatives were studied. To show the general applicability of this method, various aryl aldehydes were efficiently reacted with 2-naphthol and amide derivatives in the same conditions. The results are summarized in Table 1. The effect of electron and the nature of substituents on the aromatic ring did show expected strong effects in terms of yields under these reaction conditions. Aromatic aldehydes containing electron-withdrawing groups (such as nitro groups) or electron-donating groups (such as methoxy groups) were employed and they were found to react well to give the corresponding amidoalkyl naphthol in good to excellent yields. Aromatic aldehydes having electron-withdrawing groups on the aromatic ring (Table 1, entries 7, 21, 22, 23 29, 30) react faster than electron-donating groups (Table 1, entries 6, 14, 15, 26).
Synthesis of amidoalkyl naphthol derivatives catalyzed by [2-MPyH]OTf under thermal solvent-freea.
| Entry | Aryl aldehyde | R | M.p (°C) | Time (min) | Yield (%)b [Reference] |
| 1 | Benzaldehyde | NH2 | 173–175 | 30 | 92 [21] |
| 2 | 2-Chlorobenzaldehyde | NH2 | 149–151 | 20 | 94 [17] |
| 3 | 4-Chlorobenzaldehyde | NH2 | 169–171 | 15 | 95 [21] |
| 4 | 2,4-Dichlorobenzaldehyde | NH2 | 141–143 | 15 | 95 [17] |
| 5 | 4-Bromobenzaldehyde | NH2 | 168–170 | 20 | 94 [21] |
| 6 | 3-Methoxybenzaldehyde | NH2 | 241–243 | 20 | 93 [18] |
| 7 | 3-Nitrobenzaldehyde | NH2 | 185–187 | 10 | 97 [21] |
| 8 | 1-Naphthaldehyde | NH2 | 164–166 | 15 | 95 [17] |
| 9 | Benzaldehyde | CH3 | 228–230 | 45 | 91 [21] |
| 10 | 2-Chlorobenzaldehyde | CH3 | 195–197 | 35 | 92 [21] |
| 11 | 4-Chlorobenzaldehyde | CH3 | 236–238 | 30 | 94 [20] |
| 12 | 2,4-Dichlorobenzaldehyde | CH3 | 225–227 | 30 | 93 [19] |
| 13 | 4-Bromobenzaldehyde | CH3 | 231–233 | 30 | 93 [22] |
| 14 | 3-Methoxybenzaldehyde | CH3 | 203–205 | 35 | 92 [23] |
| 15 | 4-Methoxybenzaldehyde | CH3 | 164–166 | 30 | 93 [20] |
| 16 | 2,5-Dimethoxybenzaldehyde | CH3 | 252–254 | 35 | 92 [16] |
| 17 | 3,4,5-Trimethylbenzaldehyde | CH3 | 193–195 | 45 | 91 [19] |
| 18 | 2-Methylbenzaldehyde | CH3 | 202–204 | 45 | 93 [24] |
| 19 | 4-Methylbenzaldehyde | CH3 | 225–227 | 30 | 94 [20] |
| 20 | 4-Dimethylaminobenzaldehyde | CH3 | 82–84 | 20 | 96 [25] |
| 21 | 2-Nitrobenzaldehyde | CH3 | 181–183 | 30 | 94 [25] |
| 22 | 3-Nitrobenzaldehyde | CH3 | 241–243 | 30 | 94 [21] |
| 23 | 4-Nitrobenzaldehyde | CH3 | 236–238 | 20 | 96 [20] |
| 24 | Benzaldehyde | Ph | 234–236 | 35 | 91 [21] |
| 25 | 4-Chlorobenzaldehyde | Ph | 174–176 | 20 | 95 [21] |
| 26 | 3-Methoxybenzaldehyde | Ph | 168–170 | 30 | 93 [19] |
| 27 | 3,4,5-Trimethylbenzaldehyde | Ph | 237–239 | 35 | 91 [19] |
| 28 | 4-Methylbenzaldehyde | Ph | 191–193 | 30 | 94 [21] |
| 29 | 3-Nitrobenzaldehyde | Ph | 216–218 | 20 | 96 [21] |
| 30 | 4-Nitrobenzaldehyde | Ph | 244–246 | 15 | 96 [26] |
a Aryl aldehyde (1 mmol), 2-naphthol (1 mmol), amide derivatives (1.2 mmol), [2-MPyH]OTf as a catalyst (5 mol%).
b Isolated yield.
To find out the optimum quantity of [2-MPyH]OTf, the reaction of 2-naphthol, benzaldehyde, and acetamide was carried out under thermal solvent-free conditions using different quantities of [2-MPyH]OTf (Table 2). Ionic liquid [2-MPyH]OTf as a catalyst of 5 mol% gave excellent yield in 45 min as can be seen from Table 2. A slight excess of the acetamide was found to be advantageous and hence the molar ratio of 2-naphthol to acetamide was kept at 1:1.2. To optimize the temperature in the mentioned reaction, we have carried out a model study with benzaldehyde and 2-naphthol and acetamide using 5 mol% of catalyst at various temperatures under solvent-free conditions. Table 2 clearly demonstrates that 125 °C is an effective temperature in terms of reaction time and yield obtained.
Optimization of the amount of [2-MPyH]OTf and the reaction temperature for the preparation of N-((2-hydroxynaphthalen-1-yl)(phenyl)methyl)acetamide (Table 1 entry 9)a.
| Entry | Catalyst amount (mol%) | Temperature (°C) | Time (min) | Yield (%)b |
| 1 | 1 | 125 | 180 | 60 |
| 2 | 5 | 100 | 90 | 85 |
| 3 | 5 | 125 | 45 | 91 |
| 4 | 10 | 100 | 90 | 85 |
| 5 | 10 | 125 | 45 | 91 |
| 6 | 15 | 125 | 45 | 91 |
a Benzaldehyde (1 mmol), 2-naphthol (1 mmol), acetamide (1.2 mmol).
b Isolated yield.
The reusability of the catalysts is an important advantage and makes them useful for commercial applications. The catalyst plays a crucial role in the success of the reaction in terms of the rate and the yields [29]. For example, benzaldehyde reacted with 2-naphthol and acetamide in the presence of 5 mol% ionic liquid catalyst to give the N-((2-hydroxynaphthalen-1-yl)(phenyl)methyl)acetamide in 91% yield under thermal solvent-free conditions after 45 min of reaction time. After completion of the reaction (monitored by TLC), the product was extracted with CH2Cl2 and the catalyst was recovered from the aqueous layer. Ionic liquid catalyst is more soluble in water than in CH2Cl2 solvent. The catalyst could be reused five times for the synthesis of analogues product without significant loss of activity. The results were summarized in Table 3.
Reusability studies of catalysts for the synthesis of N-((2-hydroxynaphthalen-1-yl)(phenyl)methyl)acetamide (Table 1 entry 9)a.
| Catalytic run | Fresh | 1 | 2 | 3 | 4 |
| Isolated yield (%)b | 91 | 91 | 90 | 89 | 89 |
a Benzaldehyde (1 mmol), 2-naphthol (1 mmol), acetamide (1.2 mmol), [2-MPyH]OTf as a catalyst (5 mol%).
b Isolated yield.
In Scheme 2, we propose a possible mechanism for the synthesis of amidoalkyl naphthol derivatives in the presence of [2-MPyH]OTf as a catalyst. The reaction of 2-naphthol with aromatic aldehydes in the presence of ionic liquid as a catalyst is known to give ortho-quinone methides (o-QMs). The same o-QMs, generated in situ, have been reacted with amide to form amidoalkyl naphthol derivatives. A reasonable explanation for this result can be given by considering the nucleophilic addition to o-QMs intermediate favorable via conjugate addition on the α,β-unsaturated carbonyl group and finally this intermediate will aromatize to produce the final aromatic compound [30].
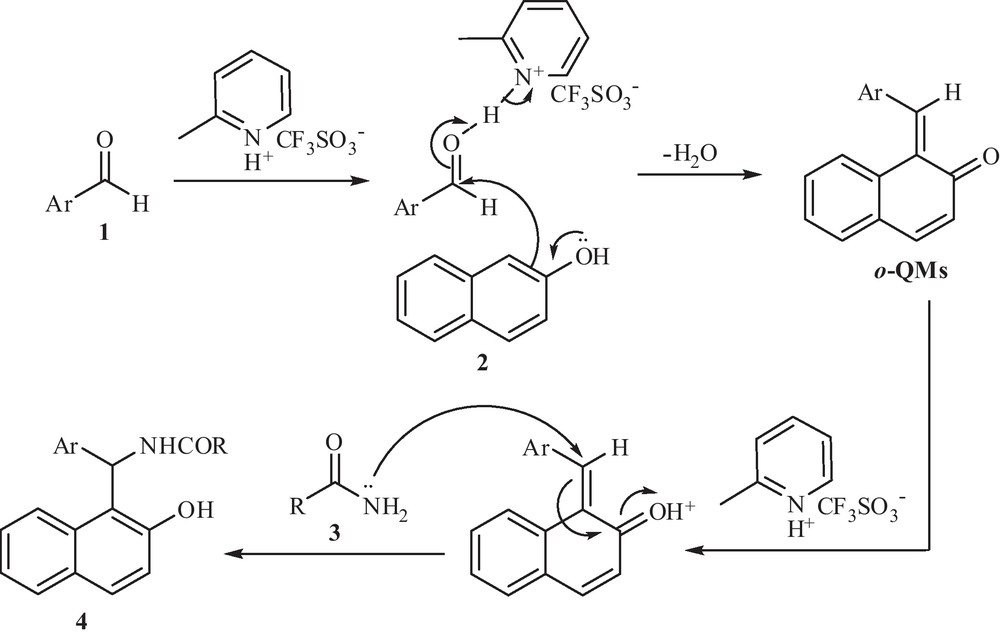
Suggested mechanism for the synthesis of amidoalkyl naphthols via conjugate addition reaction.
3 Conclusion
In summary, this article describes a green, simple and efficient protocol for the synthesis of amidoalkyl naphthol derivatives by the reaction of between aldehyde, 2-naphtol and amide in the presence of [2-MPyH]OTf. The notable merits of this protocol are cleaner reaction conditions, shorter time reaction, improved yields and simple experimental and work-up procedure. Also, the catalysts were able to be reused easily for five-time experiments with a small decrease in the catalytic activity of the recovered catalyst.
4 Experimental
All starting materials were obtained from Merck and Fluka, and were used without further purification. Melting points were obtained on a Thermo Scientific apparatus and were not corrected. IR spectra were recorded on a FT-IR Bruker (WQF-510) spectrometer. 1H and 13C NMR spectra were recorded on a Bruker DRX-400 AVANCE spectrometer (400 and 100 MHz, respectively). Mass spectra were recorded on an Agilent technologies 5973 network mass selective detector (MSD) operating at an ionization potential of 70 eV.
4.1 General procedure for the synthesis of ionic liquid catalyst
The ionic liquid [2-MPyH]OTf as a catalyst was synthesized according to literature [28]. A white solid was formed in high purity and then the physical data (IR, NMR) of these known ionic liquid was found to be identical. Spectral data: IR (KBr, cm−1) 2983, 1631, 1365, 1223, 1070, 957, 887, 579; 1H NMR (400 MHz, CDCl3): δ 2.93 (s, 3H), 7.26–7.67 (m, 2H), 8.29–8.36 (m, 1H), 8.84 (d, J = 5.9 Hz, 1H), 17.21 (brs, 1H); 13C NMR (100 MHz, CDCl3): δ 154.1, 146.7, 141.3, 128.1, 125.3, 120.7, 21.1.
4.2 General procedure for the synthesis of amidoalkyl naphthol derivatives
To a mixture of aldehydes (1 mmol), 2-naphthol (1 mmol) and acetamide (1.2 mmol), effective amount of ionic liquid [2-MPyH]OTf as a catalyst was added. The mixture was stirred under thermal solvent-free condition at 125 °C in oil bath for appropriate time and the reaction was followed by TLC. After completion of reaction, mass was cooled to room temperature, then the solid residue was dissolved in ethyl acetate and the mixture stirred for 5 min. Then solvent was evaporated, the remaining solid product was recrystallized in aqueous ethanol-water.
4.3 Recycling of [2-MPyH]OTf as an ionic liquid catalyst
In case of a hydrophilic ionic liquid, i.e., [2-MPyH]OTf, the reaction mixture was diluted water and extracted with CH2Cl2 (2 * 10 mL). The combined organic extracts were washed with water, dried over anhydrous Na2SO4, concentrated under vacuum and the resulting product was purified either by recrystallization to afford pure product. The ionic liquid can be recovered either by extracting the aqueous phase with CH2Cl2 or by evaporating the aqueous layer under vacuum. The ionic liquid thus obtained was further dried at 60 °C under reduced pressure for use in subsequent runs.
4.4 Spectral data for the synthesis of amidoalkyl naphthol derivatives
4.4.1 1-((2-hydroxynaphthalen-1-yl)(3-methoxyphenyl)methyl)urea (Table 1, entry 6)
Yield: 93%; M.p 241-243 °C (lit. [18] 242–244 °C); IR (KBr, cm−1) 3485, 3407, 3371, 3175, 3067, 2957, 1649, 1601, 1527, 1435, 1067, 825, 739; 1H NMR (400 MHz, CDCl3): δ 3⋅63 (s, 3H), 5⋅87 (s, 2H), 7.17 (d, J = 8.5 Hz, 1H), 7.21 (t, J = 8.1 Hz, 1H), 7.27 (t, J = 7.6 Hz, 1H), 7.41 (t, J = 7.5 Hz, 1H), 7.53 (m, 2H), 7.77 (t, J = 8.5 Hz, 2H), 7.86 (brs, 1H), 7.97 (m, 2H), 8.59 (d, J = 8.1 Hz, 1H), 9⋅96 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 27.3, 48.5, 118.7, 119.1, 120.7, 121.5, 123.6, 127.1, 123.5, 128.7, 129.1, 130.1, 130.9, 132.4, 133.7, 145.8, 148.1, 153.7, 170.1; MS m/z = 322 (M+, 7.11%), 320 (30.27%), 276 (37.49%), 260 (65.01%), 230 (100%), 202 (55.17%), 189 (8.09%), 144 (33.58%), 127 (19.29%), 115 (36.67%), 101 (27.51%), 88 (15.87%), 77 (17.21%), 63 (12.11%), 51 (13.51%).
4.4.2 1-((2-hydroxynaphthalen-1-yl)(3-nitrophenyl)methyl)urea (Table 1, entry 7)
Yield: 97%; M.p 185-187 °C (lit. [21] 184–186 °C); IR (KBr, cm−1) 3415, 3390, 3357, 3130, 2977, 1651, 1600, 1545, 1437, 1375, 1060, 957, 741; 1H NMR (400 MHz, CDCl3): δ 5⋅97 (s, 2H), 7.18 (t, J = 8.1 Hz, 1H), 7.23 (d, J = 8.5 Hz, 1H), 7.28 (t, J = 7.7 Hz, 1H), 7.39 (t, J = 7.3 Hz, 1H), 7.57 (m, 2H), 7.71 (t, J = 8.5 Hz, 2H), 7.87 (brs, 1H), 7.97 (m, 2H), 8.61 (d, J = 8.2 Hz, 1H), 10⋅17 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 47.9, 117.8, 118.7, 120.8, 121.3, 122.7, 126.9, 128.5, 129.7, 131.1, 132.3, 133.9, 145.7, 147.9, 154.5, 170.7; MS m/z = 337 (M+, 7.28%), 336 (28.49%), 276 (18.37%), 260 (70.21%), 230 (100%), 202 (28.09%), 189 (6.51%), 145 (6.67%), 115 (12.83%), 43 (23.27%).
4.4.3 N-((2-hydroxynaphthalen-1-yl)(phenyl)methyl)acetamide (Table 1, entry 9)
Yield: 91%; M.p 228-230 °C (lit. [21] 229–230 °C); IR (KBr, cm−1) 3397, 3241, 3057, 2955, 1637, 1580, 1375, 1337, 1057, 898, 745; 1H NMR (400 MHz, CDCl3): δ 1.97 (s, 3H), 7.09 (m, 1H), 7.13 (m, 1H), 7.17 (m, 1H), 7.21 (m, 1H), 7.20 (m, 2H), 7.23 (m, 1H), 7.27 (m, 1H), 7.35 (t, J = 7.6 Hz, 1H), 7.74 (d, J = 9.3 Hz, 1H), 7.79 (d, J = 7.9 Hz, 1H), 7.87 (s, 1H), 8.46 (d, J = 8.6 Hz, 1H), 10.03 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 23.1, 40.3, 119.3, 119.7, 123.1, 123.8, 126.4, 126.8, 127.5, 128.3, 128.9, 129.2, 129.7, 131.9, 143.2, 155.7, 169.3; MS m/z = 305 (M+, 21%), 246 (29.16%), 245 (50.57%), 231 (100%), 232 (31.18%), 202 (16.11%), 115 (10.05%).
4.4.4 N-((2-hydroxynaphthalen-1-yl)(3-methoxyphenyl)methyl)acetamide (Table 1, entry 14)
Yield: 92%; M.p 203-205 °C (lit. [23] 202–204 °C); IR (KBr, cm−1) 3395, 3261, 2957, 1657, 1605, 1545, 1437, 1065, 879, 739; 1H NMR (400 MHz, CDCl3): δ 2.01 (s, 3H), 3.65 (s, 3H), 7.15 (t, J = 8.1 Hz, 1H), 7.17 (d, J = 8.5 Hz, 1H), 7.23 (t, J = 7.6 Hz, 1H), 7.36 (t, J = 7.5 Hz, 1H), 7.52 (m, 2H), 7.75 (t, J = 8.5 Hz, 2H), 7.81 (brs, 1H), 7.97 (m, 2H), 8.59 (d, J = 8.1 Hz, 1H), 10.15 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 23.3, 33.5, 48.9, 118.7, 119.1, 120.7, 121.9, 123.3, 123.9, 127.2, 128.7, 129.1, 130.2, 130.9, 132.4, 133.5, 145.7, 148.1, 153.8, 169.5; MS m/z = 322 (M+, 8.01%), 321 (32.23%), 278 (5.01%), 261 (49.18%), 247 (15.32%), 231 (100%), 218 (12.07%), 202 (7.25%), 189 (9.38%), 134 (9.31%) 115 (11.17%), 109 (4.03%) 43 (14.06%).
4.4.5 N-((2-hydroxynaphthalen-1-yl)(4-nitrophenyl)methyl)acetamide (Table 1, entry 23)
Yield: 96%; M.p 236-238 °C (lit. [20] 237–238 °C); IR (KBr, cm−1) 3397, 3265, 2978, 2573, 1647, 1615, 1535, 1437, 1357, 1063, 877, 739; 1H NMR (400 MHz, CDCl3): δ 2.03 (s, 3H), 7.21 (d, J = 8.1 Hz, 1H), 7.25 (d, J = 8.7 Hz, 1H), 7.29 (t, J = 7.7 Hz, 1H), 7.43 (t, J = 7.5 Hz, 1H), 7.53-7.59 (m, 2H), 7.83 (t, J = 9.3 Hz, 2H), 7.89 (d, J = 7.2 Hz, 1H), 8.07 (m, 2H), 8.63 (d, J = 8.1 Hz, 1H), 10.13 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 22.7, 47.9, 117.9, 118.5, 120.8, 121.4, 122.7, 126.9, 128.7, 129.5, 130.1, 132.3, 133.9, 146.1, 147.9, 153.3, 170.1; MS m/z = 336 (M+, 26.67%), 319 (75.97%), 276 (52.03%), 260 (54.11%), 231 (63.83%), 202 (45.13%), 230 (100%), 115 (18.02%).
4.4.6 N-((2-hydroxynaphthalen-1-yl)(3-nitrophenyl)methyl)benzamide (Table 1, entry 29)
Yield: 96%; M.p 216-218 °C (lit. [21] 214–216 °C); IR (KBr, cm−1) 3395, 3267, 2975, 1657, 1615, 1535, 1460, 1075, 957, 748; 1H NMR (400 MHz, CDCl3): δ 7.15 (t, J = 8.3 Hz, 1H), 7.18 (d, J = 8.3 Hz, 1H), 7.23 (t, J = 7.3 Hz, 1H), 7.41 (t, J = 7.5 Hz, 1H), 7.54 (m, 2H), 7.69 (d, J = 8.2 Hz, 2H), 7.74 (t, J = 8.5 Hz, 2H), 7.89 (m, 1H), 7.97 (m, 2H), 8.07 (d, J = 8.2 Hz, 2H), 8.61 (d, J = 8.1 Hz, 1H), 9⋅17 (brs, 1H), 10⋅43 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 47.7, 117.9, 118.7, 120.5, 121.3, 122.7, 126.5, 127.8, 128.4, 128.7, 129.3, 129.7, 132.3, 132.8, 133.5, 145.4, 147.6, 153.8, 169.7; MS m/z = 398 (M+, 12.27%), 381 (49.51%), 276 (26.87%), 260 (34.71%), 246 (6.53%), 230 (52.67%), 202 (24.03%), 115 (11.31%), 105 (100%), 77 (54.17%), 51 (7.57%).
Acknowledgement
This research was supported by the make inquiries commission of the University of Payame Noor, Sari, Iran.


