1 Introduction
The ruthenium complex cis-RuL2(SCN)2 with L = 2,2′-bipyridyl-4,4′-dicarboxylatoacid (bpca), have been reported to sustain 108 electron transfer cycles allowing a hypothetical life time of a dye-sensitization solar cell (for a recent review see [1]) of 20 years [2]. More recent photocurrent imaging experiments in which only part of solar cells were illuminated with light from a solar simulator however showed clear photodegradation patterns [3–5]. Since temperature effects could be excluded only the ruthenium complex had a potential ability to undergo photochemical reactions, it had to be concluded that the effective turn-over number achievable with ruthenium complexes adsorbed to TiO2 is rather of the order of 107 [6]. This would sustain an efficient dye-sensitization solar cell only for approximately 2 years. However, several circumstances make the interpretation of the photodegradation of this ruthenium complex more difficult. First it was observed that photodegradation at the start of illumination was surprisingly large and gradually decreasing with time. Second, even if the entire dye-sensitization cell was homogeneously illuminated, photodegradation did only occur in certain areas, while it was delayed in others. Third, some solar cells remained remarkably stable over a longer period of time. Fourth it was observed that photodegradation was significantly reduced under open circuit condition of the dye-sensitization solar cell [5]. The interpretation suggested was that the nature of surface states near the oxidized ruthenium complex is critically determining the photochemical stability. The higher the current efficiency, the larger the instability and the chance for a photooxidation of the sensitizer. It is the oxidized sensitizing molecule, which is the critical element for instability, depending on the bonding to the TiO2 nanoparticles. It may either react rapidly in an irreversible way or it may remain stable until it is being regenerated by tri-iodide ions. The aim of this work is, to underline and reinvestigate this hypothesis by determining oxidized products of the sensitizer via high-pressure liquid chromatographic (HPLC) techniques.
2 Experimental
2.1 Dye-sensitization solar cells
Dye-sensitization solar cells were prepared as previously described [3–5]. The nanostructured TiO2-layer (particle size approximately 15 nm) was prepared from DEGUSSA TiO2 powder (P25) by spreading a viscose paste onto an FTO-conductive glass. The layers prepared were comparatively thin (approximately 5–8 μm), which limited the obtained solar energy efficiency to 2–4% under 1.5 AM simulated solar light (the solar simulator was a sulfur lamp activated by microwaves which has negligible contribution of ultraviolet light). The electrolyte was acetonitrile with 0.5 mol I3–, 50 mmol I2. Tertbutylpyridine was added to improve the photovoltage. The sensitizer used was RuL2(SCN)2, with L = bpca. It was purchased from SOLARONICS.
The counter electrode was a platinum covered conductive glass electrode. At 80 °C, the TiO2 electrode was put into the ethanol solution containing the ruthenium sensitizer. Under this condition, the sensitizer bonded to the TiO2 nanoparticles. The sealing of dye-sensitization solar cells was accomplished with Syrline from 3 M Company. The dye-sensitization cell was completed until a small opening was left through which a vacuum was applied to the cell. This vacuum was allowed to suck in the electrolyte. Then the opening was closed with melted Syrline.
2.2 Photocurrent imaging
Images of photocurrent were obtained with a set-up which was previously described [3]. With the dye-sensitization solar cell attached to a scanning table photocurrent measurements were performed with a laser spot point by point. During these experiments, the light intensity of the laser spot did typically not exceed solar light intensity. For every measured point only a few seconds were needed. The computer assembled the information to an overall photocurrent image.
2.3 High-pressure liquid chromatography
The instruments used were a Merck–Hitachi pump L6200, a Merck–Hitachi auto sampler AS2000A and the Waters 990 diode array detector. HPLC columns of the company Knaur were employed (RP8). The eluate used was a solution of acetonitrile (Merck, Uvasol) and diluted sulfuric acid (5 × 10−4 mol, Merck).
2.4 General experimental observations
Several dye-sensitization cells were initially tested during a period of 9 days while maintained in the dark. Typically a decrease of solar energy efficiency by 1% (which, for example, means from 4% to 3%) was observed. Power out-put measurements indicated that the photocurrent density was effected, not the photovoltage output. This means that some interfacial rearrangements of adsorbed sensitizer molecules on TiO2 particles may have occurred.
3 Experimental results
3.1 Photodegradation experiments with the pure sensitizer solution
It is known from photochemistry literature that ruthenium complexes in solution may not sustain more than 20–100 electron transfer processes before degrading in an irreversible way [9]. When the ruthenium complex was dissolved in 1 mol NaOH, which displaced the absorption spectrum towards shorter wavelength compared to the spectrum in ethanol solution (in ethanol the Ru complex has three maxima at 305, 377 and 503 nm and the solution exposed in a cuvette to the light of a solar simulator, the reddish color changed within 4 days to dark gray. When TiO2 particles were added, the color change was observable after 1 day with the discoloring of the solution. TiO2 particles apparently accelerate the photochemical degradation. The chromatogram of the ruthenium complex in 1 mol NaOH solution after 4 days of solar simulator illumination is shown in Fig. 1a.
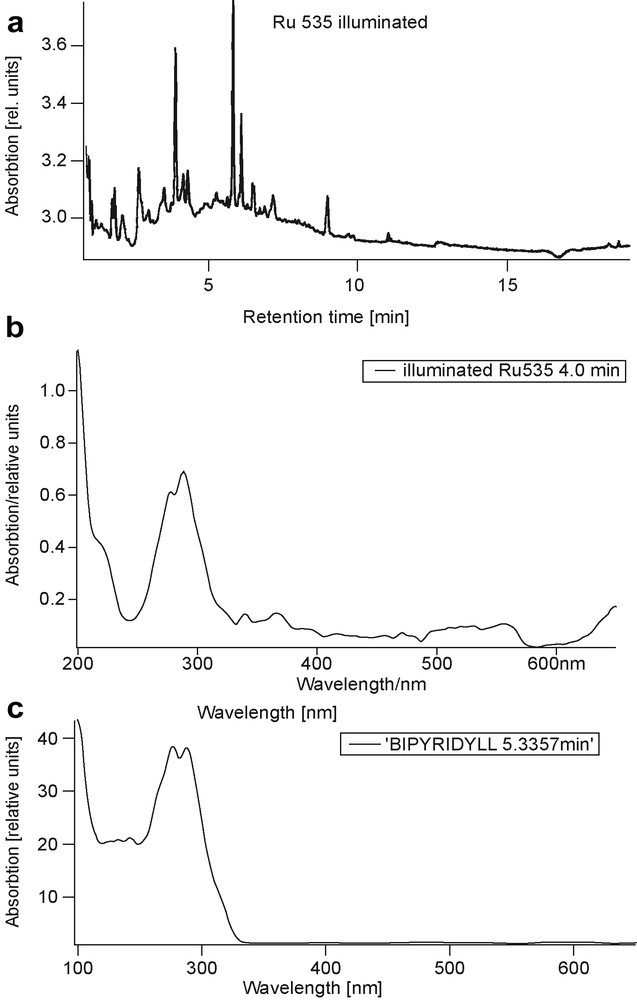
a) Chromatogram of a cis-RuL2(SCN)2 with L = 2,2′-bipyridyl-4,4′-dicarboxylatoacid (bpca) in 1 M NaOH illuminated for 4 days. b) Absorption spectrum of species with retention time of 4.0 min. c) Absorption spectrum of bipyridyl, (retention time of 5.33 min).
A multitude of chemical compounds is detected within a detention time of 15 min. As an example, the spectral absorption of the compound with a retention time of 4.0 min is shown in Fig. 1b. It may be compared with the absorption spectrum of the bipyridyl ligand of the ruthenium complex (shown in Fig. 1c).
However, when measured independently, this bipyridyl ligand only appeared with a retention time of 5.33 min, which indicates that the species shown in Fig. 1b may not be identical altogether, the experiment of illuminating the ruthenium complex in homogeneous solution underlines the high photochemical reaction activity of the ruthenium complex. When allowed to interact with TiO2 particles without the possibility of forming strong bonds, no improvement of stability is observed.
3.2 Selectively illuminated dye-sensitization cells
When dye-sensitization cells are illuminated through a circular mask and a photocurrent image is taken, the area of illumination is clearly perceived as an area of decreased photocurrent efficiency in the center of the cell [3–5]. Two examples of such a selective illumination treatment are shown in Fig. 2 (in the top left corner, there is also a area of decreased efficiency due to evaporated electrolyte). First it can be seen that the initial photocurrent efficiency is quite inhomogeneous, depending on the preparation technique and the accuracy of producing an even TiO2 layer. With our cells also the non-illuminated areas lost photocurrent efficiency as seen for an illumination period of already 2 weeks. The decreased photocurrent efficiency within the illuminated area shows that some photoreaction must have gone on.

Photocurrent images of dye-sensitization solar cells, sensitized with a cis-RuL2(SCN)2 with L = 2,2′-bipyridyl-4,4′-dicarboxylatoacid (bpca). At the beginning and after 2 weeks of illumination through a rhombus shaped mask.
(a) Cell H1 before illumination and. (b) After 2-week illumination. (c) Cell G4 before illumination and. (d) After 2-week illumination.
3.3 Chromatographic studies of degradated sensitizer molecules
3.3.1 Extraction with alkaline medium
When the ruthenium complex as purchased was dissolved in ethanol and a chromatogram taken, two main peaks are observed with retention times at 0.71 and 3.46 min. This is shown in a three-dimensional spectrum (Fig. 3), which also shows that some smaller additional peaks are observed at retention times around 1 min. The spectra observed are those expected for the ruthenium complex. One is apparently dealing with two very close modifications of the ruthenium complex. Since alkaline solution was considered for a displacement reaction to desorb the attached ruthenium complex from the TiO2 nanoparticles, a test measurement was made to elucidate the effect of alkaline medium on the chromatographic performance. The TiO2 layer was sensitized with a ruthenium complex and, under exclusion of light, NaOH solution used to again dissolve the ruthenium complex from the TiO2-layer. Fig. 4a shows the chromatogram obtained and Fig. 4b compares the spectra obtained with a retention time of 0.41, 0.51, 1.06, and 1.47 min. Species with a retention time of 0.3 and 1.03 min clearly show the typical absorption spectrum of the ruthenium complex. The other two did not have this typical spectrum, especially the species with a retention time of 0.9 min. Apparently associated with the displacement reaction, the treatment is leading to a desorption of the ruthenium complex. It shows an absorption below 300 nm only. Since this intermediate also appeared among the photodegradation products, it cannot be considered as such but is a product of the detachment of the ruthenium complex. Interestingly in Fig. 4a, the intermediate with a retention time of 3.46 min as seen for the methanol solution does not any more show up as a pronounced signal.

Three-dimensional diagrams showing chromatograms of the ruthenium complex in methanol.

(a) Chromatogram of the ruthenium complex extracted from TiO2 with the aid of 1 mol NaOH. (b) Absorbtion spectra of four species taken at 0.41, 0.51, 1.06, 1.47 min retention time, respectively.
3.4 Long-term photodegradation experiments
Several long-term photodegradation experiments were performed with dye-sensitization cells, which were exposed to solar simulated light for 2 months. This corresponds to a real solar illumination time, which is five times larger, which means 10 months long. Two of these experiments will be explained here.
Fig. 5a shows the chromatogram obtained with 90 μl of a solution extracted from such an aged cell. It contained 90% acetonitrile and 10% aqueous alkaline liquid. New peaks are seen with retention times of 5, 7 and 9 min. After opening the illuminated and aged solar cells, the TiO2 nanoparticles were scratched off and the ruthenium dye dissolved with a NaOH solution, which immediately became reddish colored. Then the solution was pressed through a filter of 0.45-μm dimension. This procedure was performed in the dark in order to exclude the possibility that the already dissolved ruthenium complex could engage in a homogeneous photochemical reaction.

(a) Chromatogram and (b) five spectra (taken at 0.8; 3.3; 5.8; 7.4 and 9.4 min retention time) of the ruthenium complex and products of his degradation extracted from a dye-sensitization solar cell illuminated for 2 months, (c) magnification of 5.8, 7.4 and 9.4 min.
During typical experiments a pronounced pre-run-peak was observed (seen in Fig. 5a) at a very short retention time (0.8 min), which was characterized by the typical absorption of the ruthenium complex. It is assumed that undissolved ruthenium aggregates or 15 nm TiO2 particles with adsorbed ruthenium complex may contribute to this peak, which characterizes species, which are not retained in the HPLC column.
Fig. 5b shows spectra of peaks from Fig. 5a: the spectrum of the pre-run-peak (0.8 min), spectrum of Ru complex (3.3 min) and spectra of three degradation products (5.8; 7.4; 9.4 min).
Fig. 5c shows magnification of three peaks which have different spectrum from Ru complex.
Fig. 6a shows a chromatogram with a magnification from retention time of 4–6 min in Fig. 6b. In Fig. 6c, the spectra are shown for the species with a retention time of 4.3, 4.6 and 5.1 min, respectively. The species 4.6 and 5.1 min do not show the typical maximum at 300 nm. The species 4.3 and 4.6 min do not show the typical peaks at 540 nm. This type of spectrum was not observed in the chromatography of the original dye. We are apparently dealing with photooxidation products from the dye-sensitization solar cell. Also the numerous small peaks, which are seen in Fig. 6b have not been obtained when starting with the original ruthenium complex solution in the chromatographic procedure. Additional characteristic chromatograms of photodegradated ruthenium complexes from dye-sensitization cells were obtained. A series of different products can be identified, some of them coinciding but others not. It may be concluded, that the spectrum of products is dependent from exposure time to light and from the specific photoelectrochemical conditions of the cells involved.

(a) Chromatogram and (b) amplification for retention times between 4 and 6 min, and (c) three absorption spectra for retention times of 4.3, 4.6 and 5.1 min.
3.4.1 Extraction with acid medium
Parallel experiments have also been performed in our laboratory using acid solutions for the extraction of adsorbed ruthenium complexes from nanocrystalline dye-sensitization solar cells [7].
Four solar cells have been examined for possible products of ruthenium dye.
Chromatograms and spectra from such experiments (pp. 137 and 139 in [7]) show that, in addition to peaks obtainable from the original complex, new species appear, which have to be associated with the photodegradation process in the sensitization cell. Remarkable are the peaks between 5 and 7 min, because they appear in all four cells. In all cases, only small amounts of such products were detected, which is reasonable on account of the comparatively short duration of the illumination process.
4 Discussion
While the ruthenium complexes have a very limited photochemical stability in homogeneous solution, they significantly increase their stability when attached to TiO2 particles via two carboxilate bonds. As Fig. 7 shows, a significant bonding can occur between the ruthenium centers via the carboxilate groups to the titanium, which, in the reduced Ti(III) form, can engage in transition metal back bonding. However, on TiO2 nanoparticles, several different surfaces may participate in bonding as well as very specific surface states. It may be concluded, that the bonding conditions can strongly vary within the nano-structure of the TiO2 layer. Also the number of carboxyl groups which is interacting may be of relevance. The oxidized molecule will be exposed to a different chemical environment in dependence of the adsorption of the molecule prior to the photoreaction.
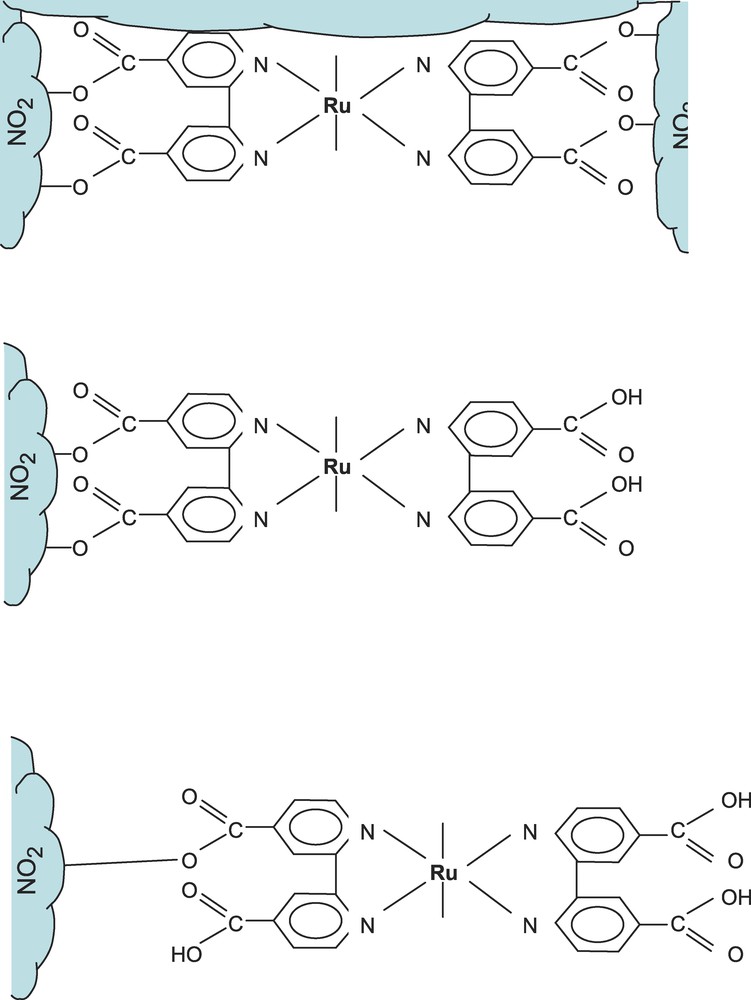
Scheme showing a structure of ruthenium complex interacting with TiO2 nanoparticles. This scheme serves to discuss the interfacial bonding situation.
The interpretation of the observed photodegradation on photocurrent images of dye-sensitization cells relates photodegradation to the varying nature of chemical bonding of the ruthenium complex to the TiO2. There are surface sites where degradation can occur very fast and there are other sites where the ruthenium complex is quite stable over a long period of time. Surface states apparently dominate the degradation kinetics. Such an effect of surface adsorption sites on the degradation kinetics of sensitizing dyes has already been recognized three decades ago [8]. The chromatographic studies with ruthenium complexes extracted from sensitization solar cells confirm that photodegradation may take place in ruthenium complex sensitized solar cells. Various species of ruthenium complex degradation have been identified without properly characterizing them. Since it cannot be assumed that all adsorption sites of the ruthenium complex on TiO2 are equally perfect, such interpretation of a surface state mediated degradation of the ruthenium sensitizer is a reasonable basis for further investigations. A key target should be, to better understand the chemistry of interfacial bonding between the ruthenium complex and the TiO2-particles and to find out under what physical conditions the bonding situation can be optimized and what TiO2-preparations provide the most ideal conditions for long-term stability of ruthenium sensitizers. As the situation may be characterized at the moment, the adsorbed ruthenium sensitizer may not be sufficiently stable to maintain dye-sensitization cells active and efficient over a period of 20 years [6]. Selected long-term experiments, which seem to contradict, can also be interpreted in such a way that a surplus of sensitizer was present to puffer degradation or the solar energy conversion efficiency was quite low, which would, of course, prolong the stability of a dye-sensitization solar cell. The results obtained here indicate that there is a research challenge towards optimizing the bonding interaction between TiO2 and the ruthenium complex towards a long-term stability of ruthenium complex sensitized solar cells.
Acknowledgements
The authors thank Dr. C.-H. Fischer for the introduction into HPLC measurements and many suggestions and discussions. They also thank Dr. A. Barkschat for critical discussions and help.


