1 Introduction
Selective oxidation of aromatic compounds is a less investigated topic compared to epoxidation of olefins and oxidation of alkanes. At the same time, the products of selective aromatic oxidation, quinones can be found in the molecular framework of numerous natural products and thus are biologically active (e.g. vitamins) [1]. This direct approach to important synthetic targets is usually accomplished by the application of stoichiometric toxic heavy metal (e.g. CrO3) and halogen-based oxidants (hypervalent iodine compounds) leading to large amounts of toxic wastes. A classical example of such an “environmentally unfriendly” process is the preparation of 2-methyl-1,4-naphthoquinone (vitamin K3, 2MQ) by stoichiometric oxidation of 2-methylnaphthalene (2MN) by CrO3 in sulfuric acid [2–5]. It should be noted that an excess of oxidant is usually used in these stoichiometric oxidations. Thus, a huge amount of Cr-containing wastes is generated (Sheldon's E factor > 18) [3,4]. Notably, the yield of 2MQ does not exceed 40–50%. The low yield of 2MQ can be explained by the occurrence of several pathways of 2MN oxidation (Scheme 1). Two quinones, 2MQ and 6-methyl-1,4-naphthoquinone always result from aromatic oxidation. Hückel calculations indicated that the coefficients of the HOMO orbital are comparable explaining why the two quinones are produced [6]. Some authors claim the only formation of 2MQ, but these data are not necessarily very reliable. Such conclusions could be derived from inaccuracy of analytical procedures, e.g. non-separation of the two quinones in GC analysis. Additionally, 6MQ is less stable than 2MQ. The quinone ratio can depend on the catalyst, in the range from 2MQ:6MQ ∼ 5:1 to about 1:1 ratio. Along with aromatic oxidation, the oxidation of methyl group often takes place. Finally, the products of the oxidative coupling of intermediate radicals and over-oxidation products are also formed.
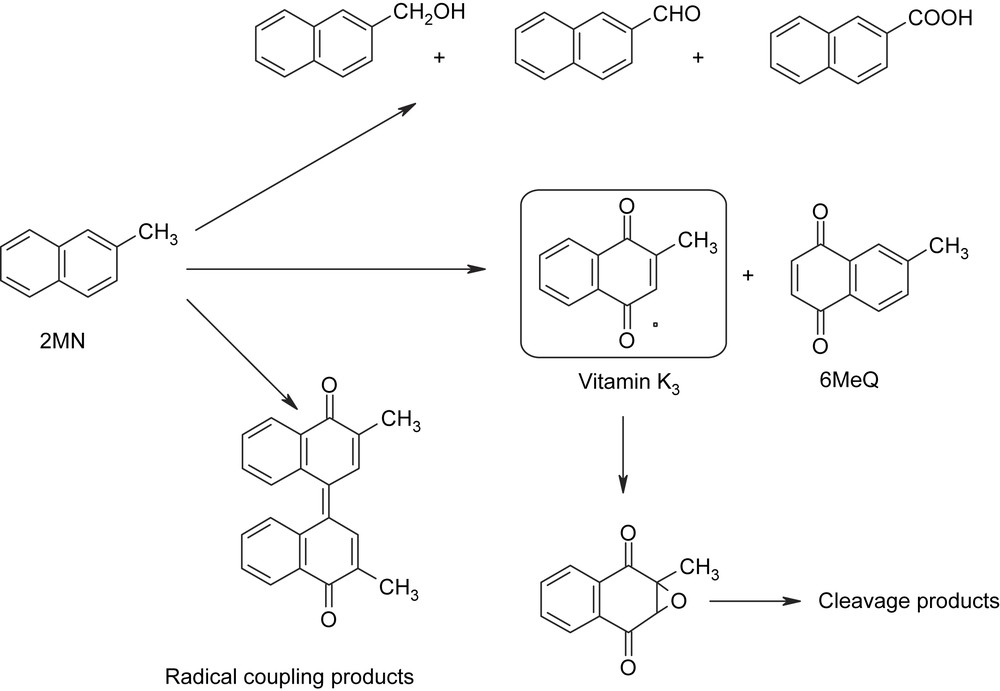
Multiple pathways in oxidation of 2MN.
Many efforts have been made to prepare 2-methyl-1,4-naphthoquinone (vitamin K3) in a selective and proper way. Several stoichiometric and catalytic approaches have been proposed for the demanding oxidation of 2MQ (for detailed discussion on the preparation of 2MQ see Refs. [7,8]). The catalytic methods using CH3ReO3 [9,10], Pd(II)-polystyrene sulfonic acid resin [11], metalloporphyrin [6,12] and metallophthalocyanine [13–18] complexes in combination with H2O2 or peroxides provided a cleaner oxidation, but the yields of target 2MQ were still moderate.
In order to avoid the formation of some by-products, 2-methyl-1-naphthol (MNL) was proposed to be a starting substrate for the synthesis of 2MQ instead of 2MN [19]. Using titanium-silicate Ti-MMM-2 catalyst and H2O2 78% yield of 2MQ has been obtained indicating the utility of this approach [7].
We have recently shown that iron tetrasulfophthalocyanine (FePcS) covalently grafted onto silica support [13–16,20,21], onto TiO2 nanoparticules [22] and immobilized on chitosan support [23] were efficient catalysts in selective oxidation of phenols [13–16, 21], alkynes [20] and β-isophorone [22,23]. Homogeneous selective oxidation of polysaccharides has been published [24]. Good catalytic activity and selectivity of FePcS-based catalysts in all these oxidations prompted us to study FePcS–SiO2 catalyst for the oxidation of MNL.
In continuation of our studies on the green catalytic oxidation applied to fine chemistry we report herein a study of the oxidation of 2MNL catalysed by supported iron phthalocyanine.
2 Results and discussion
The supported catalyst FePcS–SiO2 was prepared according to a novel approach using activation of sulfonate groups by an activating agent triphenylphosphine triflate prepared from triphenylphosphine oxide and triflate anhydride (Scheme 2) [25,26].
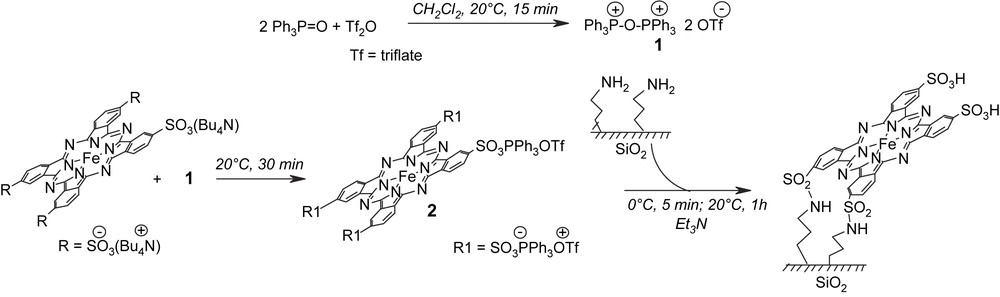
Covalent anchoring of FePcS onto amino-modified silica and schematic representation of supported catalyst.
This supported catalyst has been characterized by UV–vis spectroscopy, XPS method, BET measurements and chemical analysis [25]. Diffuse reflectance UV–vis spectrum showed Q band at 640 nm. The homogeneity of the complex anchored onto surface can be estimated from the broadness of the Q band. The full width at half maximum of the 640 nm band was about 130 nm for the supported catalyst prepared by this novel method compared to 180 nm for the supported catalyst prepared by conventional sulfochloride route [13] suggesting a more uniform distribution of the complex in the former case. XPS spectra indicated that the amino-modified silica contained the N1s signal at 399.2 eV, and the neat FePcS had a N1s signal at 398.9 eV. The XPS spectrum of the supported FePcS–SiO2 catalyst, along with the principal signal at 399.0 eV, showed a new signal at 401.6 nm. The appearance of a new signal is compatible with the formation of a covalent sulfonamide bond. A FePcS content determined by iron analysis was found to be 39 μmol/g and a BET specific surface was 185 m2 g−1.
FePcS–SiO2 supported catalyst was used in combination with tBuOOH for the oxidation of MNL. We studied the influence of different reaction parameters on the efficiency of MNL oxidation. The standard conditions used for the oxidation of MNL employed the catalyst, substrate and oxidant in the molar ratios 1:200:1000. It should be noted that oxidation was very rapid for heterogeneous reaction. After only 5 min of reaction the conversion of MNL exceeded 80% (Fig. 1).
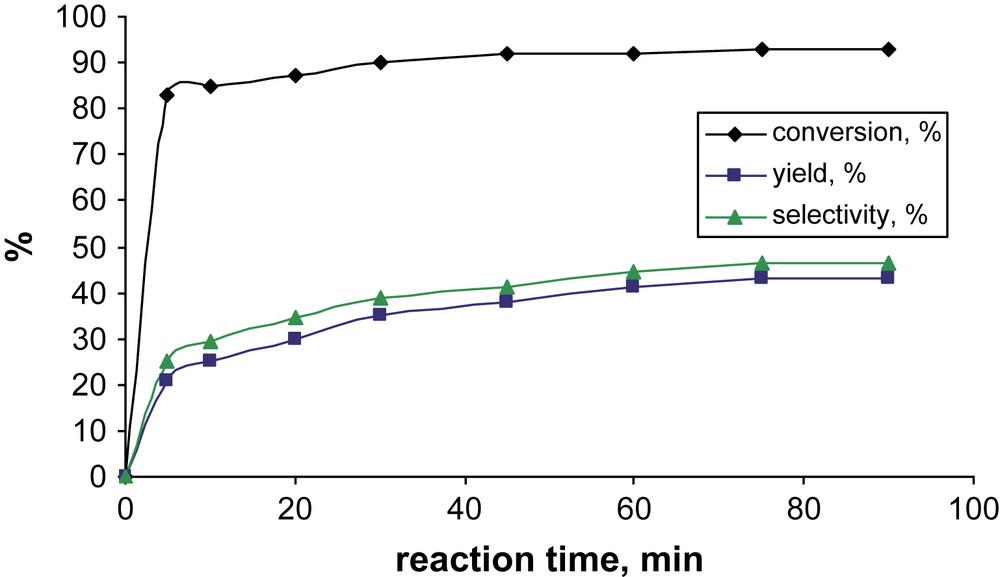
Kinetics of MNL oxidation. Reaction conditions: 0.5 mol% FePcS–SiO2, [MNL] = 0.025 M, [TBHP] = 0.125 M, 80 °C, 1 mL 1,2-dichloroethane.
However, the yield of 2MQ gradually increased achieving the maximal value at a reaction time of 1 h suggesting the formation of intermediate product(s) which could be precursor(s) of 2MQ. The dependence of the efficiency of oxidation on the reaction temperature was not strong. However, the best selectivity was obtained at 80 °C. Further experiments were performed at this temperature. In contrast, the selectivity of MNL oxidation exhibited notable dependence on the solvent nature (Fig. 2).
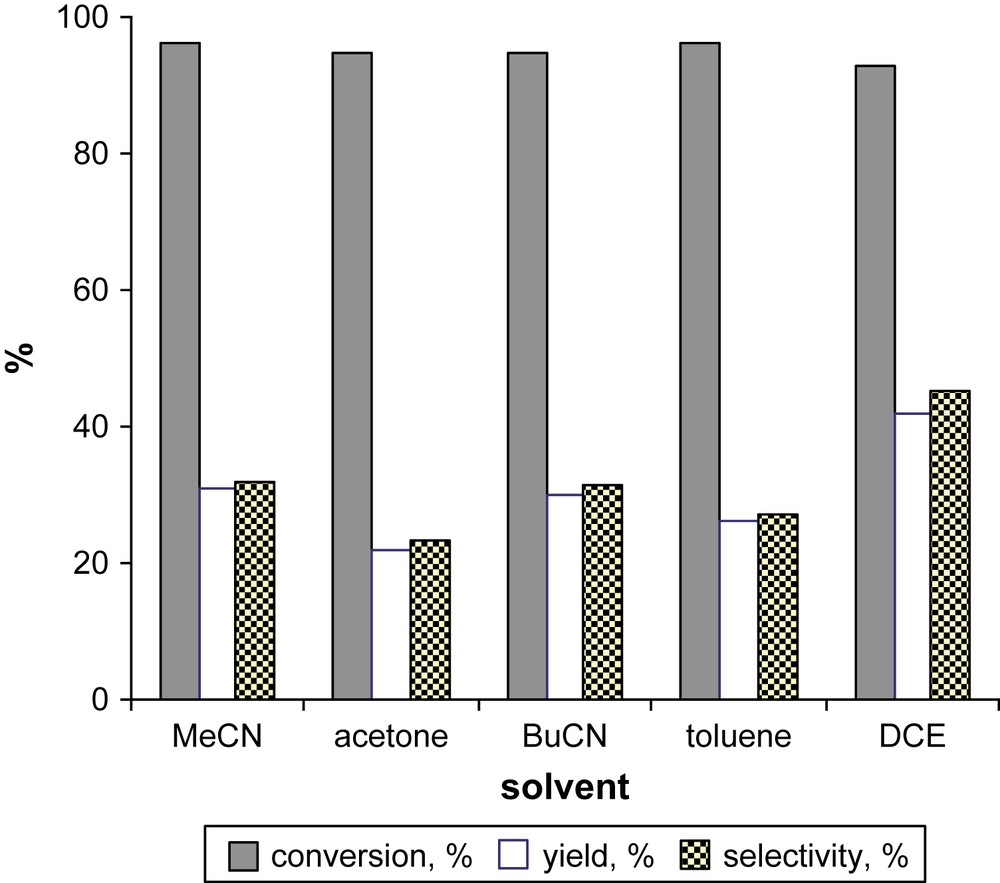
Influence of solvent on the oxidation of MNL. Reaction conditions: 0.5 mol% FePcS–SiO2, [MNL] = 0.025 M, [TBHP] = 0.125 M, 80 °C, 1 h.
The use of DCE as the solvent allowed a 93% conversion of MNL and 45% selectivity in 2MQ. It should be noted that the increase of the initial MNL concentration from 0.025 M to 0.1 M resulted in the increase of selectivity in 2MQ from 44% to 49% (Fig. 3). This finding is of importance since a higher volume yield can be obtained using more concentrated solution of substrate. We have also checked the influence of the addition of substrate (portion-wise or at once) on the selectivity of MNL oxidation. When using Ti-MMM-2–H2O2 catalytic system, portion-wise addition of MNL provided a significant increase of the selectivity of the oxidation [7]. This finding was explained by suppressing oxidative coupling due to intermediate aryloxyl radicals whose concentration was lower at a lower MNL concentration [7]. In contrast, portion-wise MNL addition in the FePcS–SiO2–TBHP system resulted in lower 2MQ yield suggesting the absence of intermediate naphthoxyl radicals in the course of MNL oxidation. This is supported by the observed dependence of the reaction selectivity on the MNL concentration.
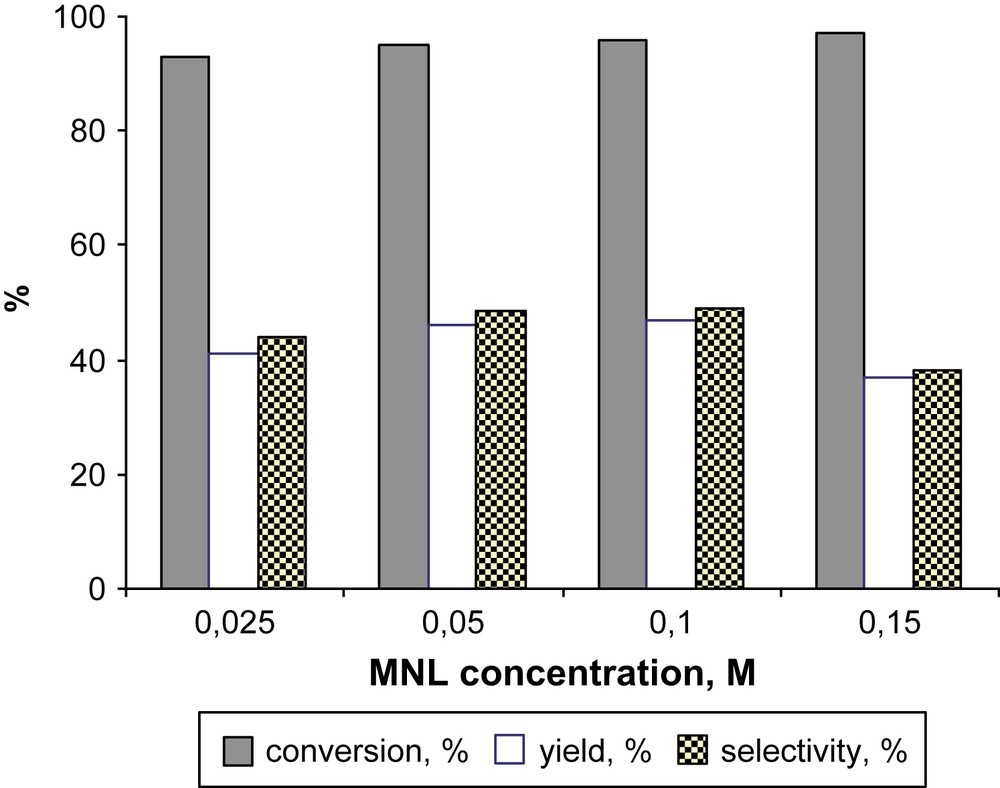
Dependence of the oxidation of MNL on the concentration of substrate in DCE. Reaction conditions: 0.5 mol% FePcS–SiO2, [MNL] = 0.025 M, [TBHP] = 0.125 M, 80 °C, 30 min.
The oxidation of phenol to quinone needs 2 equivs of oxidant. A 96% conversion of MNL was obtained with 3 equivs of TBHP, the selectivity in 2MQ being 40%. A progressive increasing of the amount of the oxidant resulted in the increasing of selectivity of MNL oxidation to 2MQ suggesting participation of TBHP in the reaction steps leading to 2MQ (Fig. 4). Using 4.5 equivs of TBHP a 59% selectivity at 95% conversion was attained. A further increase of the TBHP concentration led to decreasing selectivity, probably owing to over-oxidation reactions.
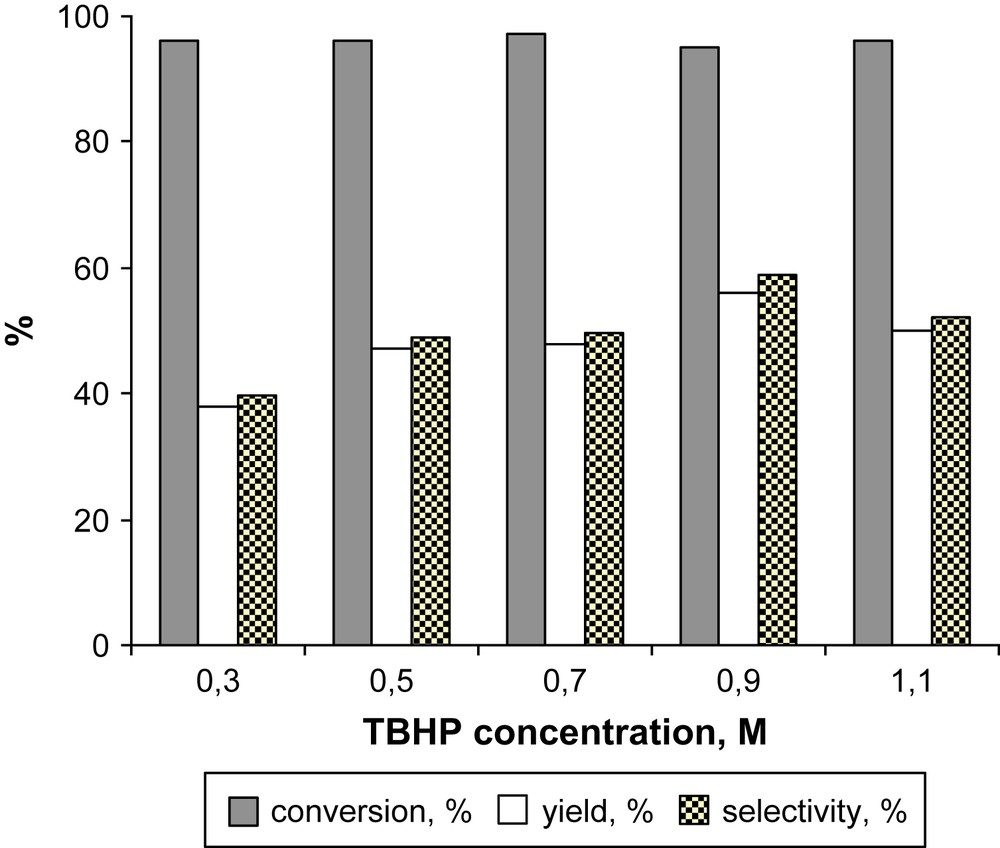
Dependence of the oxidation of MNL on the concentration of oxidant. Reaction conditions: 0.5 mol% FePcS–SiO2, [MNL] = 0.1 M in DCE, 80 °C, 1 h.
A significant advantage of this catalytic system is the small amount of catalyst needed for successful oxidation (Fig. 5). The oxidation was still efficient with only 0.25 mol% of catalyst providing 55% selectivity. Under these conditions a turnover was found to be 212 cycles. The best selectivity of 59% at 96% conversion was obtained with 0.5 mol% catalyst.
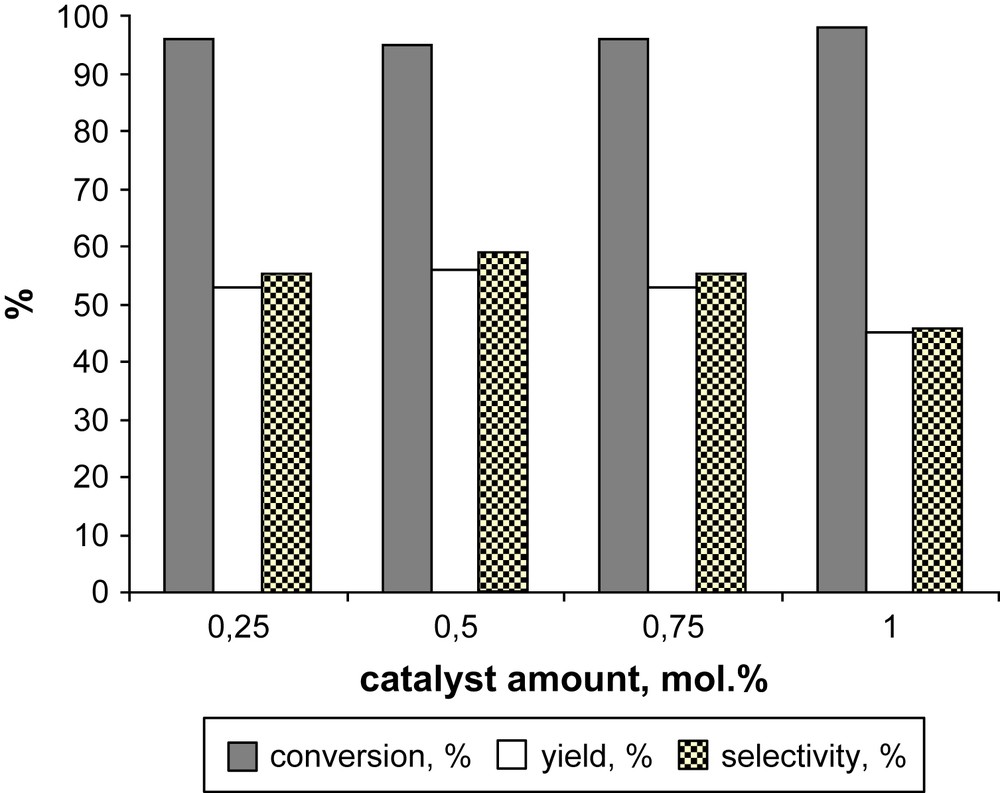
Dependence of the oxidation of MNL on the catalyst amount. Reaction conditions: [MNL] = 0.1 M in DCE, [TBHP] = 0.9 M, 80 °C, 1 h.
The recyclability of the heterogeneous catalyst was also studied under the following catalytic conditions: 0.5 mol% FePcS–SiO2, [MNL] = 0.1 M and [TBHP] = 0.5 M. After completing the first run the catalyst was isolated by filtration, washed with DCE and MeOH and dried. On reuse, we observed the same catalytic activity but a small decrease in the selectivity of oxidation.
To probe the mechanism of the phenol oxidation we have performed the oxidation of MNL in the presence of 18O2 using 18O2/Ar mixture containing 20.4% of dioxygen. The isotopic composition of products and dioxygen in the gas phase was controlled during all reaction courses for oxidation of MNL. The isotopic composition of dioxygen remained almost constant during the reaction: 99.2% 18O content in starting mixture and 98.6% 18O content after 2 h of reaction. The composition of the gas phase was also constant. These findings indicate no appreciable dioxygen formation and no dioxygen consumption during the reaction suggesting the absence of radical oxidation. Just very low 18O content (9.4 ± 0.2%) was observed in only one position of 2MQ. A majority of 2MQ (91.6 ± 0.2%) contained no 18O label that was consistent with involvement of iron based active species and the absence of long-living phenoxyl radicals. Otherwise, significant 18O content in MNQ should be found since radicals formed via 1e− oxidation of phenol can easily react with dioxygen. The absence of phenoxyl radicals is also consistent with high quinone yield while coupling products should be obtained in opposite case. Recently, we have obtained a similar very low 18O incorporation in the products of oxidation of anthracene, 2,3,6-trimethylphenol and xanthene [21].
3 Conclusion
A clean and efficient procedure for the oxidation of 2-methyl-1-naphthol to 2-methylnaphthoquinone (vitamin K3) is described using iron phthalocyanine supported catalyst and tBuOOH. The oxidation of 2-methyl-1-naphthol includes several reaction pathways as indicated in Scheme 1 for the oxidation of 2-methylnaphthalene, except the formation of isomeric 6-methyl-1,4-naphthoquinone. Obviously, the selectivity of the formation of vitamin K3 is far from 100%. However, by optimisation of the reaction conditions we were able to achieve 59% selectivity in vitamin K3 at 95% conversion which is a good result for this demanding oxidation. The advantages of the catalytic system are the short reaction time, small amount of catalyst and relatively high concentration of substrate that allows achieving a high volume yield. Labelling study indicates that the reaction most likely proceeds via non-radical mechanism involving FePcS-based active species. Although further work is still required to optimise this system in terms of selectivity and to improve its recyclability the results already obtained provide the basis for clean access to vitamin K3 by heterogeneous oxidation. Further research is in progress in our laboratories in order to understand the mechanism of oxidation that should allow improving the catalytic system.
4 Experimental section
4.1 Materials
2-Methyl-1-naphthol (98%) and 2-methyl-1,4-naphthoquinone were purchased from Aldrich and used as received. tBuOOH (Aldrich) was used as 70% aqueous solution. All the other reactants were obtained commercially and used without further purification.
Iron tetrasulfophthalocyanine, sodium salt (FePcS) was prepared according to the modified method of Weber and Busch [27,28]. The replacement of sodium ion with tetrabutylammonium cation was performed as follows. Four equivs of (Bu4N)OH (3.4 mL of 40% aqueous solution) were added to a solution of FePcS (1.5 g, 1.3 mmol) in 50 mL of water. Tetrabutylammonium salt of FePcS was extracted with CH2Cl2 (5 × 100 mL). The organic fraction was dried, the solvent removed and the product was dried overnight in vacuum at 50 °C.
4.2 Methods
1H NMR spectra were obtained using a AM 250 Bruker spectrometer. Nitrogen sorption isotherm analysis was performed using a Catasorb apparatus. The diffuse reflectance UV–vis spectra of solid catalysts were recorded on a Perkin–Elmer Lambda 9 spectrophotometer. XPS measurements were performed with an Escalab 200R (VG Scientific) spectrometer using the monochromated KαAl radiation as excitation source. The complex loading was determined by iron analysis using inductively coupled plasma-mass spectrometry method. The reaction products were identified by GC–MS (Hewlett Packard 5973/6890 system; electron impact ionization at 70 eV, He carrier gas, 30 m × 0.25 mm crosslinked 5% PHME siloxane (0.25 μm coating) capillary column, HP-5MS).
The conversions and the yields of products were determined by GC (capillary column VF5-MS, 100% dimethylpolysiloxane, 30 m × 0.25 mm, 0.25 μm coating).
4.3 Labelling experiments
Labelling experiments were performed under an atmosphere containing 20.4% of 18O2 (99.6% 18O enrichment) and 79.2% of Ar. The reaction flask contained 0.025 mmol of substrate and 0.47 mmol of 18O2 (23.5-fold excess to substrate). Isotopic compositions of products and gas phase were determined by GC–MS. Each sample was analyzed three times and m/z intensities of each peak was obtained by an integration of all scans of the peak.
4.4 Preparation of supported catalyst
A solution of triphenylphosphine oxide (620 mg, 2.25 mmol) in 10 mL of CH2Cl2 was degassed and triflate anhydride (176.5 μL, 1 mmol) was added under argon. The obtained solution was stirred at room temperature for 15 min and a solution of iron tetrasulfophthalocyanine (tetrabutylammonium salt, 486 mg, 0.25 mmol) in 20 mL of CH2Cl2 was added. The resulting solution was stirred at 20 °C for 30 min. This solution was added dropwise to the suspension of the Degussa Aerosil 200 amorphous silica modified with 3-aminopropyltriethoxysilane [13] (4.85 g) containing triethylamine (190 μl, 1.38 mmol) at 0 °C. The resulting mixture was allowed to warm to 20 °C and stirred at this temperature for 1 h. The blue solid was isolated by filtration, washed with ethanol and water and dried at 50 °C for 24 h.
Supported catalyst was characterized by chemical analysis, surface area determination and the diffuse reflectance UV–vis spectroscopy that evidence the complex grafting. The complex content was determined by metal analysis using an inductively coupled plasma-mass spectroscopy method.
4.5 General procedure for MNL oxidation
Catalytic experiments were performed under stirring in 25 mL glass vessels at 25–80 °C. The reaction was started by addition of 0.125–1.1 mmol of tBuOOH to a reaction mixture containing 0.025–0.15 mmol of MNL, 4–16 mg of the FePcS–SiO2 catalyst (0.00025–0.001 mmol of FePcS), an internal standard (biphenyl) in 1 mL of solvent. The oxidation products were identified by both GC–MS and 1H NMR techniques. MNL conversions and MNQ yields were determined by GC using biphenyl as internal standards.
Acknowledgements
This work was supported by Associated European Laboratory (‘Institut de recherches sur la catalyse', Villeurbanne, France, and Boreskov Institute of Catalysis, Novosibirsk), Russian Foundation for Basic Research and CNRS (grant RFBR-CNRS 05-03-05-03-34760). OVZ thanks the Embassy of France in Moscow for a doctoral fellowship.


