1 Introduction
Biaryl-containing molecules bind to a great diversity of proteins and therefore are found in almost any therapeutic class, for instance, oncolytics, antibiotics, or CNS and cardiovascular agents [1,2]. In particular, bridged biaryls constitute the framework of naturally occurring antimitotic compounds such as allocolchicinoids (e.g. allocolchicine 1), steganes (e.g. steganacin 2), and rhazinilam 3 [3] (Scheme 1). These molecules interact with the mitotic spindle: allocolchicinoids [4] and steganes [5] bind to tubulin at the colchicine site and inhibit the formation of microtubules, whereas rhazinilam inhibits both the disassembly and the assembly of microtubules via the formation of abnormal spiral-like structures [6]. While being structurally related, a major discrepancy exists between the three families of natural products: allocolchicinoids contain a seven-membered median ring that has enough flexibility to allow free rotation around the biaryl bond (configurationally unstable biaryl axis), thus they exist as a mixture of interconverting atropisomers at room temperature (major aR atropisomer). In contrast, steganes and rhazinilam-type molecules contain a more rigid eight- and nine-membered bridging ring, respectively, that prevents their room-temperature atropisomerization (configurationally stable axis, aR configuration). However, for all three types of compounds, the absolute configuration of the biaryl axis is a crucial parameter for tubulin-binding, since aS atropisomers do not fit the binding site and therefore are essentially inactive. The unusual structural features and potent tubulin-binding properties of allocolchicinoids, steganes and rhazinilam have been at the source of numerous synthetic studies [3]. In this account, we report on our own efforts toward the synthesis of these compounds or analogues using Suzuki–Miyaura couplings, both in non-asymmetric [7] and asymmetric [8] variants, to construct their biaryl framework.
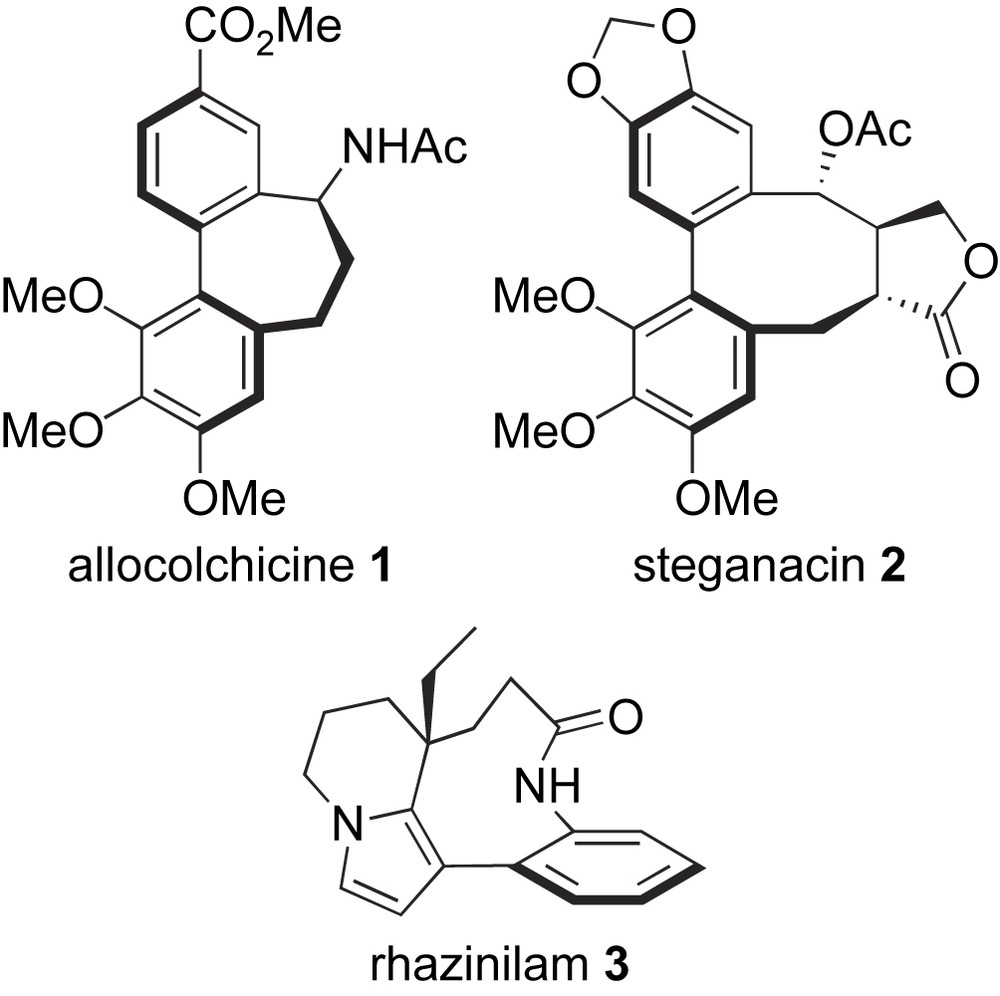
Structure of allocolchicine, steganacin, and rhazinilam.
2 Synthesis of rhazinilam analogues
2.1 The borylation–Suzuki coupling (BSC) approach to biaryls
Starting from two ortho-substituted aryl halides (Scheme 2), a variety of 2,2′-disubstituted biaryls were synthesized by a one-pot borylation–Suzuki coupling (BSC) procedure using the same palladium(0) catalyst [9]. The choice of borylation and cross-coupling components depends on the nature of the substituents present on the aromatic ring. Thus, if R1 is electron-donating and if R2 is electron-withdrawing, the borylation should be best performed on component I and the coupling of the resulting boronate with component II. Indeed, we found that the borylation reaction gives better yields with electron-rich substrates, and the resulting electron-rich boronate is more reactive for the transmetalation step of the Suzuki coupling. Besides, it is known that Suzuki couplings work better with electron-deficient halides (component II) due to an acceleration of the oxidative addition step [7]. On the contrary, if R1 is rather electron-withdrawing and R2 electron-donating, the borylation should be performed on component II and the Suzuki coupling with component I. It was found that the palladium ligand played a crucial role on the efficiency of both steps, with Buchwald's (2-dicyclohexylphosphino)biphenyl [PCy2(o-biph)] [10] giving the best results in combination to palladium(II) acetate as the metal source. In addition, a specific base had to be employed for each step, with triethylamine being suitable for the borylation step and barium hydroxide for the Suzuki coupling step.
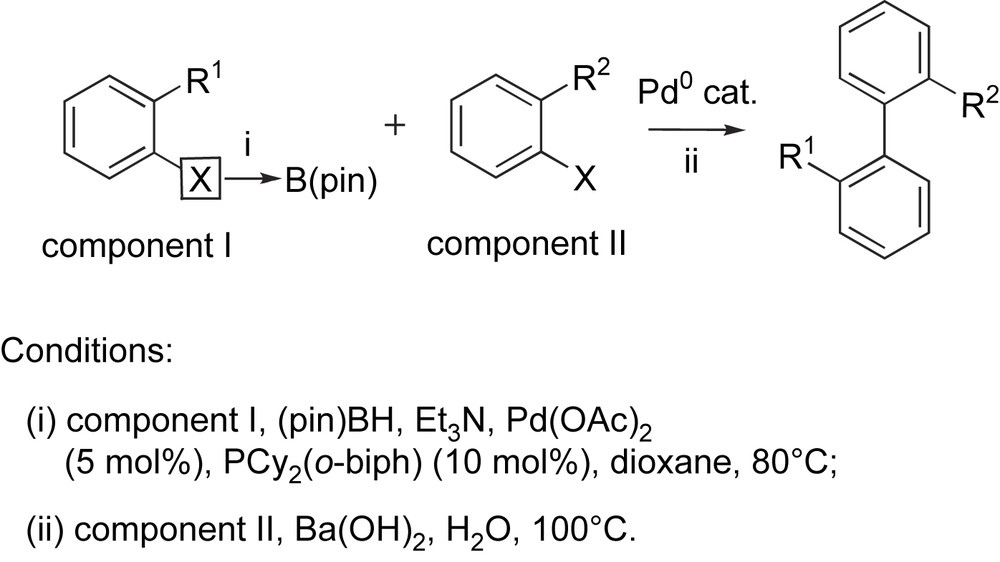
Synthesis of 2,2′-disubstituted biaryls by the one-pot BSC procedure.
The BSC procedure was applied to the synthesis of racemic analogues of rhazinilam [11] (Scheme 3). The borylation of 2-haloanilines 6, followed by coupling with iodoarene 7 (obtained in four steps from commercially available material [12]) furnished biaryls 8 that were converted in two steps into biarylcarbamate analogues of rhazinilam 4–5 bearing various electron-donating or -withdrawing R1–R3 groups. Although these molecules have a simplified structure compared to rhazinilam (3), they have very similar three-dimensional shapes. The unsubstituted racemic analogue 4a showed comparable antitubulin properties and cytotoxicities toward cancer cells to those of rhazinilam. After separation of both atropisomers of 4a on a small scale, it was shown that this biological activity is restricted to the aR atropisomer, that is twice more active than rhazinilam on the inhibition of microtubule disassembly. With this activity, (aR)-4a is still the most potent rhazinilam-type antitubulin compound known to date.
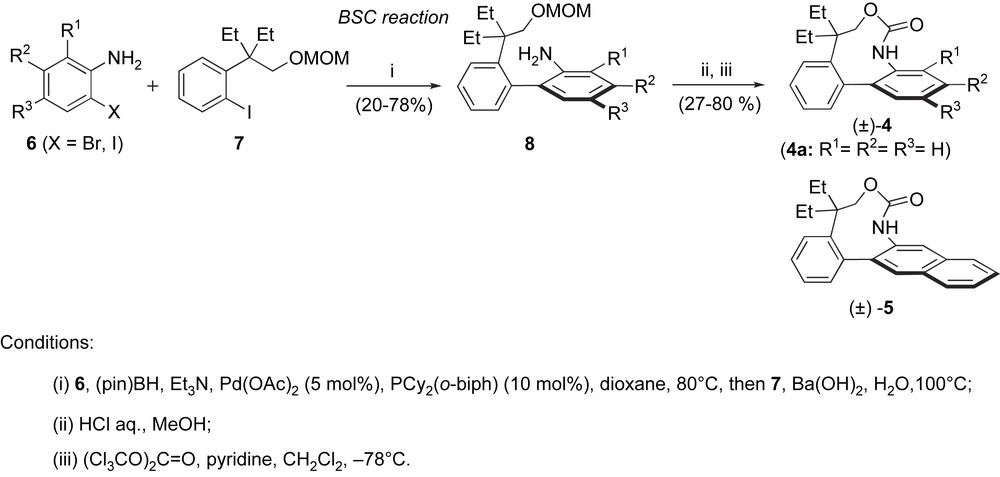
Synthesis of rhazinilam analogues by the BSC procedure.
2.2 Catalytic enantioselective synthesis of rhazinilam analogue (aR)-4a
HPLC separation of 4a racemate could provide only mg-scale quantities of (aR)-4a, that were insufficient for further biological (in vivo) evaluation. This prompted us to devise an asymmetric variant for the synthesis of the active enantiomer. In addition to the biological interest, it became clear that this would constitute a good opportunity to assess the synthetic value of enantioselective biaryl Suzuki couplings, that had not been developed to a great extent, neither in methodological studies nor in multi-step synthesis (Scheme 4) [8].
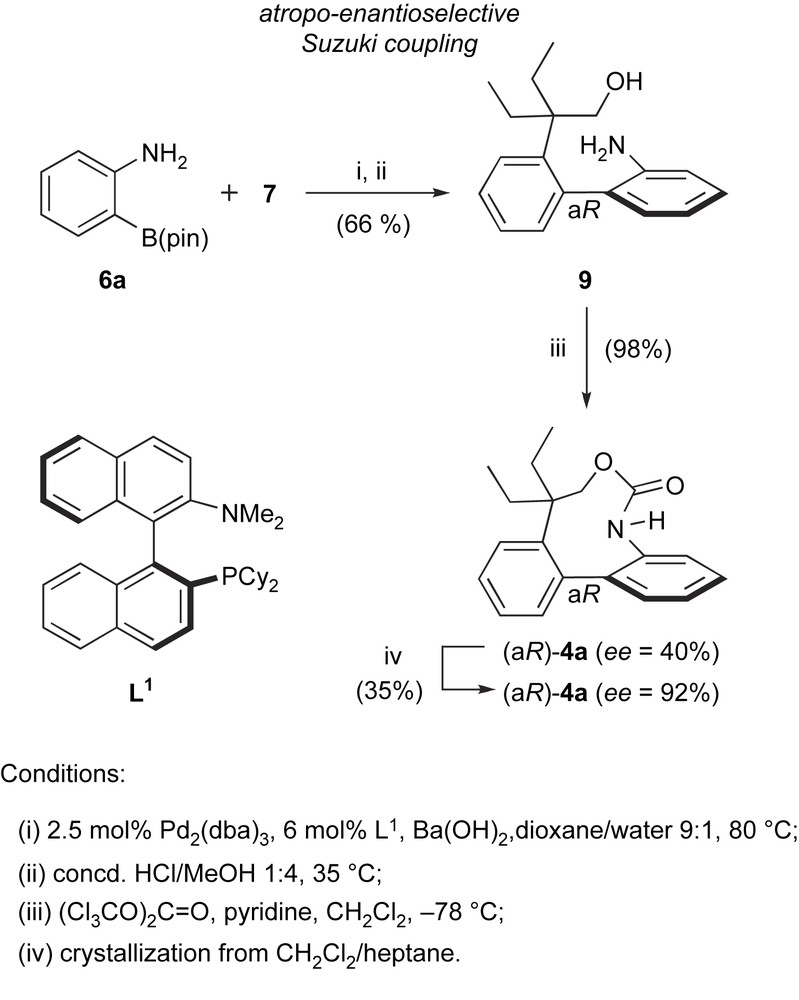
Enantioselective synthesis of the rhazinilam analogue (aR)-4a.
Aryl iodide 7 was coupled with pinacolboronate 6a under conditions similar to those used in the racemic synthesis of 4a, in the presence of 5 mol% Pd0 and 6 mol% of a chiral phosphine ligand to give, after MOM-group cleavage, aminoalcohol 9. Compound 9 and its MOM-protected precursor bear only two substituents in their 2 and 2′ positions and have therefore limited configurational stability. However, under the described conditions, the coupling was sufficiently fast (45–60 min at 80 °C) to avoid thermal epimerization of the product. Several chiral ligands were tested in this reaction, including those previously reported in other enantioselective Suzuki couplings. From this screen, Buchwald's binaphthyl-monophosphine (aS)-L1 [13] gave the highest enantioselectivity, and aminoalcohol 9 was obtained in a moderate 40% ee (and 66% yield for two steps). Cyclization with triphosgene at low temperature gave the target enantiomer (aR)-4a quantitatively and again in 40% ee. Gratifyingly, a single crystallization improved the ee to 92%, providing compound quantities suitable for further biological evaluation.
3 Asymmetric synthesis of hybrids of allocolchicine and steganacin
Allocolchicine (1) and steganacin (2) are antimicrotubule agents that both bind to tubulin at the colchicine site (Scheme 1). We envisioned to structurally combine these two families of natural products so as to obtain simple but potent inhibitors of tubulin polymerization. We took benefit of the expertise gained with the synthesis of rhazinilam analogues to design an asymmetric synthesis of such hybrid molecules. After unsuccessful attempts with enantioselective Suzuki couplings, we designed a diastereoselective variant, making use of a simple chirality induction from a benzylic stereogenic alcohol (Scheme 5) [14].
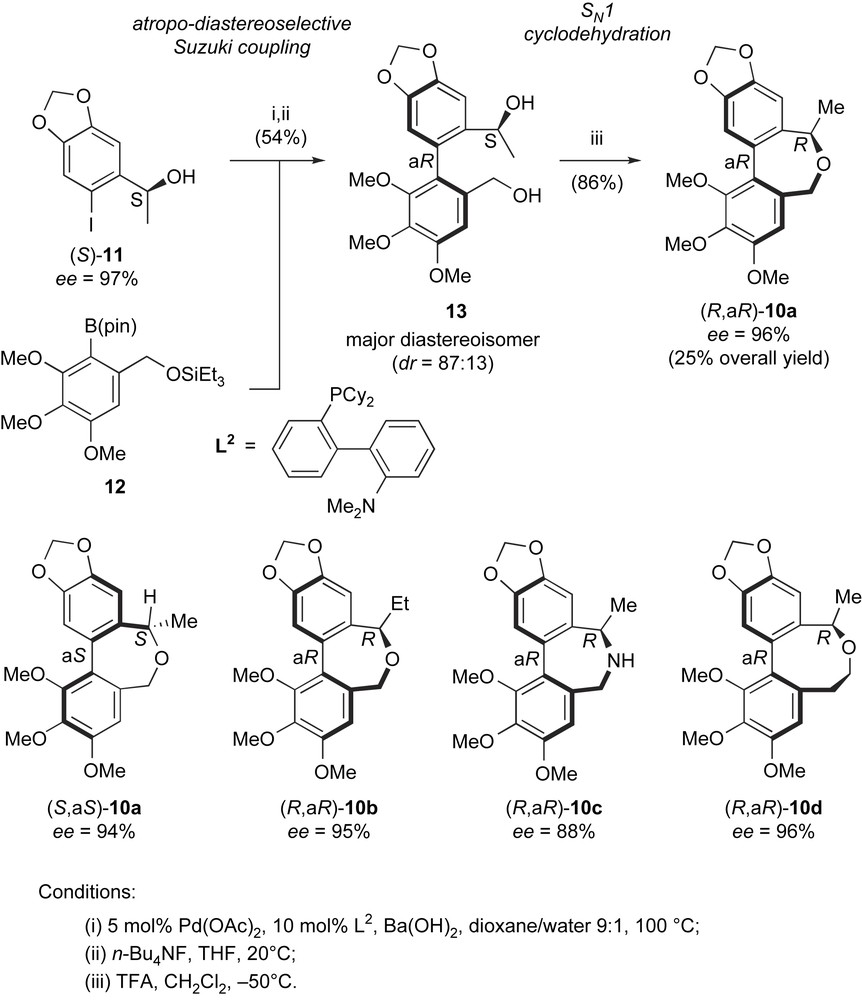
Enantioselective synthesis of allocolchicine/steganacin hybrids.
Alcohol (S)-11 was obtained in 97% ee by a Corey–Bakshi–Shibata catalytic enantioselective reduction. The coupling of (S)-11 with boronate 12 was conducted under reoptimized conditions using palladium acetate and ligand L2 as pre-catalyst. This furnished, after TES-group removal with TBAF, diol 13 as the major diastereoisomer with S,aR absolute configuration in 54% isolated yield. A good diastereoselectivity was observed in the biaryl coupling step (dr = 87:13 in favor of the S,aR diastereoisomer). A SN1-type cyclodehydration performed at −50 °C produced dibenzoxepine (R,aR)-10a with configuration inversion at the benzylic stereocenter and conservation of the optical purity of alcohol (S)-11. This can be rationalized by the formation of a configurationally stable aR-configured benzylic carbocation intermediate. Using this reaction sequence, compound (R,aR)-10a, which is very similar to allocolchicine (1) in three-dimensions, was obtained in 96% ee and 25% overall yield. As could be expected from this structural similarity, (R,aR)-10a showed colchicine-type antimicrotubule activity, which was only slightly (1.5×) inferior to that of colchicine itself. Variations in the structure of either Suzuki coupling partners 11 and 12 and in the synthetic sequence allowed the synthesis of enantiomer (S,aS)-10a and analogues (R,aR)-10b–d with similar efficiency and enantioselectivity. It could thus be checked that only the R,aR enantiomer of 10a had antimicrotubule activity, similar to other allocolchicine-type molecules. Other analogues showed interesting activities, for instance the ethyl analogue (R,aR)-10b was 1.7 times more potent than colchicine as antimicrotubule agent. In addition, analogue (R,aR)-10d, having an eight-membered median ring conformationally closer to that of steganacin-type compounds, showed a similar potency to its seven-membered ring analogue 10a. This synthetic sequence, powered by the use of an efficient and stereoselective biaryl Suzuki coupling paves the way for further structural optimization of these new types of allocolchicine/steganacin hybrids and their evaluation as new antimitotics.
4 Conclusion
We have designed modified reaction conditions for the biaryl Suzuki coupling that are specifically adapted to the synthesis of 2,2′-bridged biaryls of biological interest. Both non-asymmetric and asymmetric variants of this approach were found to be viable for the production of small libraries of molecules that act on the tubulin–microtubule equilibrium and therefore arrest mitosis. It is our hope that these studies will contribute to improve the synthesis of biaryls, that are both synthetically challenging and biologically important molecular motifs.
Acknowledgements
I thank my former colleagues at ICSN who were involved in these projects, in particular F. Guéritte, D. Guénard, and S. Thoret, as well as the graduate students who performed most experimental work: A. Herrbach, A. Décor and A. Joncour. I am also grateful to ICSN (director: J.-Y. Lallemand) for its financial support.


