1 Introduction
Concern over the use of macroalgae as a tool for monitoring heavy metal contamination in marine environments is growing [1–3]. These plant matrices can accumulate trace metals up to levels many times higher than seawater samples [4,5].
Many analytical methods have been developed for the determination of heavy metals in environmental samples [6–8]. Due to its relatively low cost and high detection limits, atomic absorption spectrometry (AAS) is among the most widely employed techniques [9–11]. This analytical method requires the solid sample to be transformed into a homogeneous liquid phase. Therefore, a sample pretreatment step is generally necessary for elemental analysis. There are several possibilities for sample treatment, such as dry ashing with a muffle furnace or wet decomposition with concentrated acid or mixture of acids at ambient pressure or in pressurized systems [12–13]. Some parameters of these procedures may influence the metals' determination by AAS. It is then important to evaluate these parameters to see their level of influence. As many variables are involved throughout the pretreatment step in the conventional procedures, experimental designs are powerful tools for the optimization of analytical procedures. Among the different groups of designs, the Plackett–Burman factorial design allows the main effects of a great number of variables to be known with relatively few experiments [14–15].
In the present work, the effects of 25 parameters on heavy metal determination in algae and the analysis of Pb, Cr and Al in real and spiked macroalgal samples are reported.
2 Experimental
2.1 Instrumentation
A muffle furnace (EHRET, Emmendingen, Germany) was used to calcinate the samples and a vibrating ball mill (Retsh MM 200, Haan, Germany), equipped with stainless steel cups and balls, was used to pulverize them.
A Shimadzu atomic absorption spectrometer (AA-680) equipped with graphite furnace (GF), deuterium-lamp background correction and gas controller and connected with a graphic printer PR-5 was used for the analysis of Pb, Cr and Al. Argon was used as a protective gas. All measurements for metals' determination were performed using hollow cathode lamps. The absorption measurements were made under conditions described in Table 1.
Operating parameters
| Furnace programs | ||||
| Step | Temperature (°C) | Ramp (s) | Hold (s) | Air flow (ml min−1) |
| Pb analysis | ||||
| Dry | 130 | 1 | 20 | 300 |
| Ash | 350 | 20 | 0 | 300 |
| Atomize | 1200 | 0 | 3 | 0 |
| Clean-out | 3000 | 1 | 1 | 300 |
| Cr analysis | ||||
| Dry | 120 | 1 | 20 | 300 |
| Ash | 700 | 20 | 0 | 300 |
| Atomize | 2000 | 0 | 4 | 0 |
| Clean-out | 3000 | 1 | 1 | 300 |
| Al analysis | ||||
| Dry | 130 | 1 | 20 | 300 |
| Ash | 700 | 30 | 0 | 300 |
| Atomize | 2400 | 0 | 3 | 0 |
| Clean-out | 3000 | 1 | 1 | 300 |
2.2 Reagents
Ultrapure water of 18.2 MΩ cm resistivity, obtained from a Milli-Q water purification system (Millipore, Bedford, MA, USA) was used for the preparation of reagents and standards. The concentrated acids HF (48%) (Merck, Darmstrad, Germay), HCl (37%) and HNO3 (65%) (CARLO ERBA, Milano, Italy) were of analytical reagent grade. Standard Pb, Cr and Al stock solutions of 1000 mg l−1 were purchased from Merck (Darmstadt, Germany) and working solutions were daily prepared by dilution of appropriate aliquots of the stock solutions. Laboratory glassware was kept overnight in 10% (v/v) HNO3 solution, and washed with deionized water before use.
2.3 Sample collection
The analytical procedure was applied to algal samples (Ulva Lactuca) collected from the Bizerte lagoon in Tunisia. This lagoon is situated near a big commercial harbor and surrounded by different industrial activities.
Macroalgae samples were carefully handpicked at 15-m depth. In order to obtain a representative sample of each sampling site, five algae subsamples distributed according to a grille of 100 m2 area were collected. These samples were mixed to form a homogeneous product. Once homogenized, the samples were transported to the laboratory and stored in polyethylene bags at −20 °C. They were defrosted, dried, and ground with a vibrational ball mill for 5 min.
2.4 Digestion procedures
2.4.1 Factors and experimental field
Twenty-five parameters, which could potentially affect the metal determination in algae, were retained after a bibliographical review of the various seaweed treatment procedures [16–18]. The majority of factors and the experimental field were selected from the combination of the following three digestion methods (Table 2).
Factors and experimental field
| Symbols | Factors | Experimental field | |
| Low level (−) | High level (+) | ||
| U1 | Oven drying temperature | 70 °C | 105 °C |
| U2 | Oven drying duration | 16 h | 48 h |
| U3 | Cooling with desiccator | Yes | No |
| U4 | Type of grinding | Mortar | Ball mill |
| U5 | Furnace heating temperature | 400 °C | 450 °C |
| U6 | Furnace heating duration | 2 h | 8 h |
| U7 | Cooling 1 | Yes | No |
| U8 | Nitric acid 1 | Yes | No |
| U9 | Hotplate dry heating 1 | Yes | No |
| U10 | Calcination 1 | Yes | No |
| U11 | Cooling 2 | Yes | No |
| U12 | Nitric acid 2 | Yes | No |
| U13 | Furnace heating 2 | Yes | No |
| U14 | Cooling 3 | Yes | No |
| U15 | Hydrochloric acid 1 | Yes | No |
| U16 | Hotplate heating 2 | Yes | No |
| U17 | Filtration | Yes | No |
| U18 | Washing 1 | Yes | No |
| U19 | Calcination 2 | Yes | No |
| U20 | Hydrofluoric acid | Yes | No |
| U21 | Hotplate dry heating 3 | Yes | No |
| U22 | Hydrochloric acid 2 | Yes | No |
| U23 | Washing 2 | Yes | No |
| U24 | Cooling 4 | Yes | No |
| U25 | Hydrochloric acid 3 | Yes | No |
Method I [19] utilizes several stages to extract metals from the studied matrix. It was performed as follows: plant material is finely crushed, homogenized and dried for 16 h at a temperature of 70–80 °C. After cooling for 30 min in the desiccator, 2 g of sample are weighed in a capsule of platinum and dried in furnace for 2 h until temperature reaches 450 °C. The obtained ashes are cooled and wetted with 2–3 ml of deionized water and 2–3 ml of concentrated HCl. The resulting solution is heated on hotplate until appearance of the first vapours. It is then filtered, with filter without ashes, in a 100-ml volumetric flask and rinsed three or four times with warm deionized water. The ashless filter paper and its contents are incinerated in the capsule for 30 min at a temperature of 550 °C. Five milliliters of HF are then added to the ashes and the obtained solution is heated to dryness on soft hotplate or water bath at a maximum temperature of 250 °C. The resultant powder is treated with 1 ml of concentrated HCl and warm deionized water. Finally, the solution is transferred into the 100-ml volumetric flask and made up to volume with deionized water after cooling.
Method II: this method was proposed by Jackson [20]. It involves the heating of the plant material for several hours in a muffle furnace at a temperature of 400–450 °C. The obtained ashes are cooled and treated with an excess of HNO3 (1 M). The resulting solution is then evaporated to dryness on a hotplate and calcined for 10 min at 400 °C, cooled and recuperated with an appropriate diluted acid in a volumetric flask.
Method III: Lozano et al. [12] utilize the following stages to treat the algal samples: samples are homogenized and 25 g are dried for 24 h at a temperature of 105 °C. Once dry, the specimens are crushed and the resulting powder is put in porcelain melting pots and then in a muffle furnace. The algae are heated gradually up to 400–450 °C for not less than 8 h. This temperature is maintained until the powder becomes whitish. Finally, 15 ml of 25% HCl (Merck, Darmstadt, Germany, Certified SAA) are added and the resulting solution is directly analyzed by SAA-E.
2.5 Plackett–Burman designs
A Plackett–Burman design, with 28 experiments for 25 factors, was carried out as a screening approach to find the significant factors affecting the responses.
The experiments, presented in Table 3, were executed in random order. For every experiment a reagent blank was also prepared. All measurements were run in triplicate for the sample and standard solutions. The average concentration value (n = 3) for every experiment is registered in Table 2, where YPb is the response representing the mean concentration of lead; YCr is the response representing the mean concentration of chromium; YAl is the response representing the mean concentration of aluminium.
Experimental plan and results
| Experimental plan | Responses (μg g−1) (n = 3) | |||||||||||||||||||||||||||
| Experience | U1 | U2 | U3 | U4 | U5 | U6 | U7 | U8 | U9 | U10 | U11 | U12 | U13 | U14 | U15 | U16 | U17 | U18 | U19 | U20 | U21 | U22 | U23 | U24 | U25 | YPb | YCr | YAl |
| 1 | 105 | 16 | No | Ball mill | 450 | 8 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | No | No | No | Yes | No | Yes | No | No | 0.09 | 0.94 | 2.99 |
| 2 | 105 | 48 | Yes | Ball mill | 450 | 8 | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes | Yes | No | No | No | No | Yes | No | 0.78 | 1.09 | 4.65 |
| 3 | 70 | 48 | No | Ball mill | 450 | 8 | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | No | Yes | No | Yes | No | Yes | No | No | Yes | 4.38 | 1.12 | 9.86 |
| 4 | 70 | 16 | Yes | Ball mill | 400 | 8 | No | No | No | Yes | Yes | No | Yes | No | Yes | Yes | Yes | No | No | Yes | No | No | No | Yes | No | 4.93 | 1.02 | 6.46 |
| 5 | 70 | 16 | Yes | Ball mill | 450 | 6 | No | No | No | No | Yes | Yes | Yes | Yes | No | No | Yes | Yes | No | No | Yes | Yes | No | No | No | 0.54 | 0.94 | 4.12 |
| 6 | 70 | 16 | Yes | Mortar | 450 | 8 | No | No | No | Yes | No | Yes | No | Yes | Yes | Yes | No | Yes | Yes | No | No | No | Yes | No | Yes | 1.57 | 0.98 | 2.04 |
| 7 | 105 | 48 | No | Mortar | 400 | 6 | No | Yes | No | Yes | Yes | No | Yes | Yes | No | Yes | No | Yes | No | Yes | No | No | Yes | No | No | 2.06 | 1.00 | 4.26 |
| 8 | 105 | 48 | No | Mortar | 400 | 6 | No | No | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | No | No | No | Yes | No | No | Yes | Yes | 1.34 | 0.91 | 3.49 |
| 9 | 105 | 48 | No | Mortar | 400 | 6 | Yes | No | No | Yes | No | Yes | Yes | No | Yes | No | Yes | Yes | Yes | No | No | Yes | No | No | No | 0.77 | 1.00 | 2.94 |
| 10 | 105 | 48 | Yes | Ball mill | 400 | 8 | No | Yes | No | No | Yes | No | No | No | No | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes | 0.87 | 1.06 | 3.59 |
| 11 | 70 | 48 | No | Ball mill | 450 | 6 | No | No | Yes | No | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | No | 3.04 | 1.13 | 9.11 |
| 12 | 105 | 16 | No | Mortar | 450 | 8 | Yes | No | No | Yes | No | No | No | No | No | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | 1.86 | 0.92 | 3.92 |
| 13 | 105 | 16 | No | Ball mill | 450 | 6 | No | Yes | No | Yes | Yes | Yes | No | Yes | No | No | No | No | Yes | Yes | No | Yes | No | Yes | Yes | 0.81 | 1.01 | 7.02 |
| 14 | 105 | 48 | Yes | Mortar | 450 | 8 | No | No | Yes | Yes | Yes | Yes | No | No | Yes | No | No | No | No | Yes | Yes | Yes | Yes | No | No | 3.04 | 1.01 | 7.92 |
| 15 | 70 | 48 | No | Ball mill | 400 | 8 | Yes | No | No | Yes | Yes | Yes | Yes | No | No | No | No | No | Yes | No | Yes | No | Yes | Yes | Yes | 0.85 | 1.07 | 5.42 |
| 16 | 105 | 16 | No | Ball mill | 400 | 8 | No | No | Yes | No | No | No | Yes | Yes | Yes | No | Yes | No | Yes | Yes | No | Yes | Yes | No | Yes | 1.39 | 1.11 | 5.2 |
| 17 | 105 | 48 | Yes | Ball mill | 450 | 6 | Yes | No | No | No | No | No | Yes | Yes | Yes | No | No | Yes | No | Yes | Yes | No | Yes | Yes | Yes | 1.29 | 1.13 | 7.53 |
| 18 | 70 | 48 | No | Mortar | 450 | 8 | No | Yes | No | No | No | No | Yes | Yes | Yes | Yes | No | No | Yes | No | Yes | Yes | No | Yes | No | 1.73 | 0.95 | 2.07 |
| 19 | 70 | 48 | Yes | Mortar | 400 | 8 | Yes | Yes | No | No | No | Yes | No | Yes | No | No | Yes | No | No | Yes | No | No | No | No | Yes | 1.07 | 0.91 | 4.67 |
| 20 | 70 | 16 | No | Ball mill | 400 | 6 | No | Yes | Yes | Yes | No | No | No | No | Yes | No | No | Yes | No | No | Yes | No | No | No | Yes | 1.21 | 1.01 | 1.98 |
| 21 | 105 | 16 | Yes | Mortar | 450 | 6 | Yes | No | Yes | No | Yes | No | Yes | No | No | Yes | No | No | Yes | No | No | No | No | No | Yes | 0.87 | 0.86 | 1.42 |
| 22 | 70 | 16 | No | Mortar | 450 | 6 | Yes | Yes | No | No | Yes | No | No | No | Yes | No | Yes | No | Yes | Yes | Yes | No | Yes | No | No | 3.42 | 0.99 | 4.4 |
| 23 | 105 | 16 | Yes | Mortar | 400 | 8 | No | Yes | Yes | No | No | Yes | Yes | No | No | No | No | Yes | Yes | Yes | Yes | No | No | Yes | No | 1.32 | 0.95 | 3.76 |
| 24 | 70 | 48 | Yes | Ball mill | 400 | 6 | Yes | No | Yes | Yes | No | No | No | Yes | No | Yes | No | No | Yes | Yes | Yes | Yes | No | No | No | 2.36 | 1.05 | 5.94 |
| 25 | 70 | 16 | No | Mortar | 400 | 8 | Yes | No | Yes | No | Yes | No | No | Yes | No | No | No | Yes | No | No | No | Yes | Yes | Yes | No | 0.42 | 0.89 | 0.25 |
| 26 | 105 | 16 | Yes | Ball mill | 400 | 6 | Yes | Yes | No | No | No | Yes | No | No | Yes | Yes | No | No | No | No | No | Yes | Yes | Yes | No | 1.76 | 1.01 | 3.1 |
| 27 | 70 | 48 | Yes | Mortar | 450 | 6 | No | Yes | Yes | Yes | No | No | Yes | No | No | No | Yes | No | No | No | No | Yes | Yes | Yes | Yes | 0.38 | 0.89 | 1.98 |
| 28 | 70 | 16 | Yes | Mortar | 400 | 6 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 3.30 | 1.05 | 4.38 |
The effect of the factor i can be estimated from bi:
The theoretical response at Xi = 0 can be estimated from b0:
To increase the response, the factor i must be maintained [21]
- - at the high level (+) if bi is positive;
- - at the low level (−) if bi is negative.
2.6 Software
All data treatment has been performed using Nemrod-W® [22] by LPRAI (Marseilles, France).
3 Results and discussion
The bi values calculated for Pb, Cr and Al are shown in Figs. 1–3.
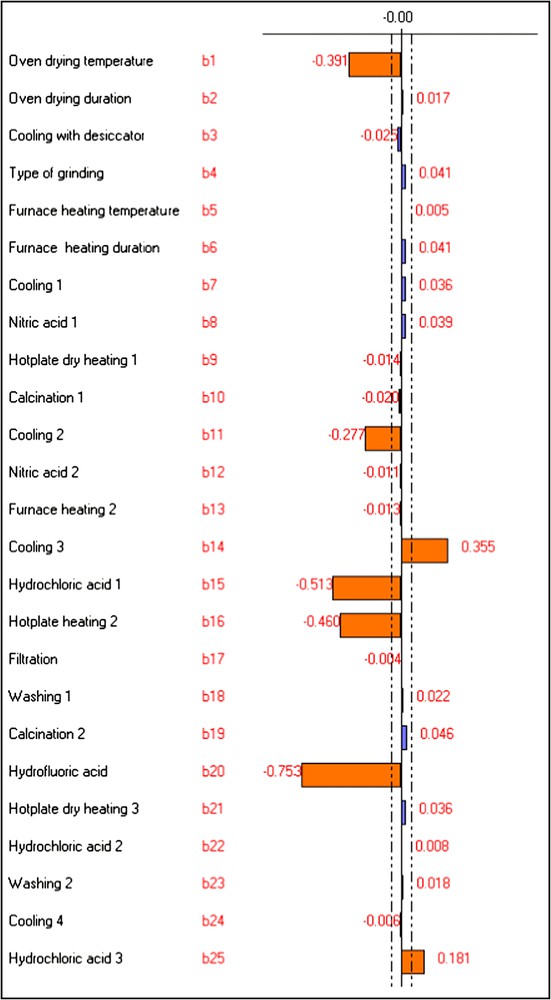
Graphic study of the factors' effects on YPb.
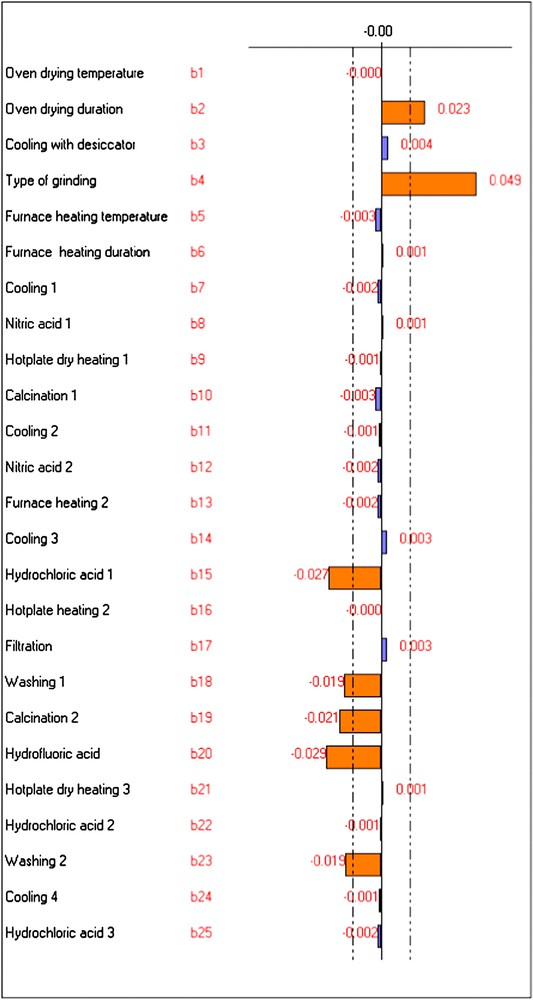
Graphic study of the factors' effects on YCr.
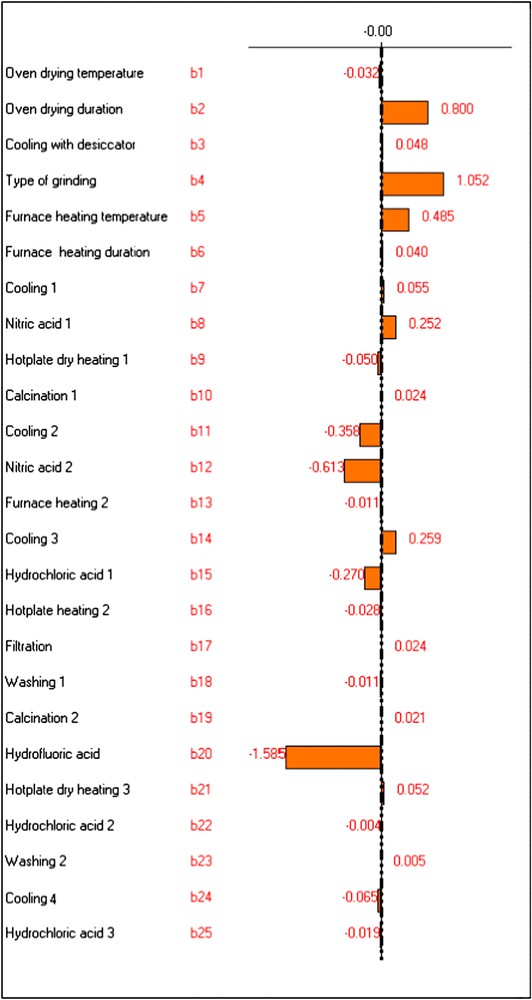
Graphic study of the factors' effects on YAl.
According to the Lenth [23] approach, every factor having a coefficient bi greater than the values delimited by the dotted lines in Figs. 1–3 (obtained by NEMROD software program) can be considered as an influent factor. The other factors have a low probability to be considered as influent.
3.1 Effect of oven drying
Among the three studied metals, the “oven drying temperature” was influential only on the determination of Pb. Its effect was negative. Consequently, the minimum level of this factor was selected for all metals' determination, i.e. alga would be dried at 70 °C instead of 105 °C.
The increase of temperature allows a faster drying of algae but risks to cause overheating phenomena in the drying oven [24]. This could cause a partial volatilization of certain metals and decrease their concentration in algae.
The “oven drying duration” was influential on the analysis of Cr and Al. It caused a positive effect on their analytical signals in algae. Therefore, the maximum level was chosen, i.e. algae would be dried for 48 h rather than 16 h.
The algae could not be completely dried for 16 h, which would have caused losses in the dry mass matter and then a reduction in the metals' response.
3.2 Effect of cooling with desiccator
Cooling with dessicator did not have a significant influence on the analytical signal of metals. So, to reduce the digestion time, the minimum level was selected for heavy metal analysis: algae would not be cooled with desiccator.
3.3 Effect of type of grinding
This variable had influence on the determination of Al and appeared as the most influential factor for Cr. For these two metals its effect was positive. Consequently, the maximum level of this variable was chosen, i.e. algae would be pulverised with ball mill for 5 min instead of being grinded with agate mortar.
The setting in solution of the trace elements seemed to be difficult to realize when the agate mortar was used. Moreover, the use of a mortar might cause heating which involved a partial volatilization of metals.
3.4 Effect of heating with the furnace
The factor “furnace heating temperature” was influential only in the case of Al. Its effect was positive. So, maximum level was chosen for metals' determination, i.e. the temperature of the furnace would be 450 °C rather than 400 °C.
The complete destruction of the organic fraction related to Al seems more difficult at 400 °C than at 450 °C.
Results on the influence of the factors “furnace heating duration” and “furnace heating 2” showed that these factors act in a negligible way on the effectiveness of the algae treatment.
Therefore, minimum levels of these factors were chosen, i.e. the duration of the first furnace heating of algae would be of 2 h rather than 8 h and a second furnace heating step would be eliminated.
3.5 Effect of cooling
Results showed that cooling after the first heating or “cooling 1” factor and after washing with water or “cooling 4” factor did not have influence on the metals' determination. Hence, these steps would be eliminated from the pretreatment of algae.
The influences of the factors "cooling 2" and "cooling 3" were important, in the case of Pb and Al. Nevertheless, their effects were opposed: negative for the first variable and positive for the second. Consequently, minimum level of the factor “cooling 2” and maximum level of the factor “cooling 3” were chosen, i.e. samples would be cooled till ambient temperature after the calcination step, but they would not be cooled after the second heating with the furnace.
The intervention of the hydrochloric acid after the calcination step could explain the positive effect of the factor “cooling 3”. Indeed, the attack of the matrix alga by hydrochloric acid is more significantly hot than cold [19].
3.6 Effect of nitric acid
From the three chosen metals, the factors “nitric acid 1” and “nitric acid 2” had significant influences only on the signal of Al. However, their effects were opposite: positive for the first and negative for the second. Consequently, maximum level was chosen for the variable “nitric acid 1” and minimum level for “nitric acid 2”, i.e. in the opposite of the nitric acid (0.2 M), the addition of nitric acid (1 M) would be eliminated from the experimental protocol.
The concentration of nitric acid and the moment of its addition may influence the determination of Al in the studied matrix. This result could be explained by the formation of insoluble Al oxides that decreased the metal's analytical signal, when nitric acid (1 M) was added.
3.7 Effect of hydrochloric acid
The influence of the factor “hydrochloric acid 1” was significant for all the studied metals. Its effect was negative on their responses.
The factor “hydrochloric acid 2” did not influence the metals' determination.
A positive significant effect of the variable “hydrochloric acid 3” was observed only for Pb detection.
Consequently, minimum levels were fixed for the variable “hydrochloric acid 1” and maximum level for variables “hydrochloric acid 2” and “hydrochloric acid 3”, i.e. concentrated hydrochloric acid would be added only one time and diluted hydrochloric acid would not be added in the final step of the pretreatment of macroalgae.
Hydrochloric acid dissolves several metal carbonates, peroxides and alkali hydroxides. Most metal form soluble chlorides, except Ag, Hg, Ti and Pb [25].
3.8 Effect of hydrofluoric acid
The factor “hydrofluoric acid” was very influential on the determination of the studied metals, especially in the case of Al and Pb. Its effect was negative on the analytical signal of all metals. So, the factor “hydrofluoric acid” was fixed at its minimum level, i.e. hydrofluoric acid would be added in the digestion of alga.
It is well established that the addition of hydrofluoric acid completes the digestion of environmental matrices by releasing trace metals included in the silicated phases [26,27]. Certain metals, such as Pb and Al, have a real affinity towards these sites which makes them resistant to any other form of acid dissolution [28]. On the other hand, hydrofluoric acid increases the solubility and stability of several metals [26].
3.9 Effect of calcination
The “calcination 1” variable did not have significant effect on metal responses, so to reduce the digestion time maximum level was selected, i.e. no calcination would take place for 10 min at 400 °C in the protocol of digestion.
The “calcination 2” factor had significant negative effect only on the Cr response. So, minimum level was selected for this variable, i.e. calcination would be integrated for 30 min at 550 °C in the macroalgal digestion.
Cr forms very stable complexes with the algal organic matter [29]. Therefore, its liberation in solution might require a durable heating in the furnace.
3.10 Effect of heating on hotplate
The factors “hotplate dry heating 1” and “hotplate dry heating 3” did not have a significant influence on the effectiveness of the macroalgal treatment.
The factor “hotplate heating 2” had a negative effect on the response of Pb.
Consequently, as opposed to the factor “hotplate heating 2”, minimum levels were chosen for the factors “hotplate dry heating 1” and “hotplate dry heating 3”, i.e. heating on hotplate until the appearance of the first vapours would be added to the treatment step, whereas heating algae in the hotplate until dryness would be eliminated from the protocol of digestion.
Dry evaporation is used to prevent the contaminations by strong and concentrated acids [30].
However, dry heating can involve losses by fusion of insoluble silicic compounds or adsorption on the walls of the containers [24].
3.11 Effect of filtration
The stage of filtration eliminates turbidity and ensures a better homogeneity of the digest but can involve losses by adsorption on the filter paper [31].
In our case, the stage of filtration did not have a significant effect on the metals' responses. Thus, this stage would be eliminated from the treatment of algae.
3.12 Effect of washing
The influence of the factors “washing 1” and “washing 2” appeared especially in the case of Cr. Their effect was negative on the responses of this element. Then, these factors were fixed at their minimum levels, i.e. the washing step would contribute to the metal analysis in alga.
Washing allows recovering the volume of solution adsorbed on the surface of containers after having emptied them. Hence, it should improve the quality of the metals' analysis.
3.13 Method suggested for the pretreatment of algae
The plant material is dried in the oven at a temperature of 70 °C during 48 h and grinded for 5 min with a vibrating ball mill. Two grams of sample are weighed in a capsule of platinum and dried in furnace for 2 h until a temperature of 450 °C. The obtained ashes are cooled and wetted with 3 ml of HNO3 (0.2 M) and 3 ml of concentrated HCl. The resulting solution is heated on hotplate until appearance of the first vapours, treated with warm deionized water and calcined in the capsule for 30 min at a temperature of 550 °C. Five milliliters of HF are added to the ashes and the obtained solution is treated with warm deionized water. Finally, it is transferred into the 100-ml volumetric flask and made up to volume with deionized water.
3.14 Validation of the proposed method
The accuracy of the proposed method was tested with the Community Bureau of Reference (BCR) certified reference material CRM 279 (sea lettuce). Two blank solutions were treated in the same way as the reference material (Table 4). Results were in agreement with the reference values and the standard deviations were low, proving a good repeatability of the proposed method. In the case of Cr and Al, recovery tests were carried out on the certified reference material, as values were not certified for both metals. The obtained results were satisfactory, demonstrating the efficiency of the suggested pretreatment method (Table 5).
Validation of the pretreatment method against BCR-CRM 279 sea lettuce (μg g−1 (d.w))
| Metal | Certified value | Found value (n = 5) |
| Pb | 13.48 ± 0.36 | 13.91 ± 0.59 |
| Cr | 10.7a | 9.62 ± 0.41 |
a Indicative value.
Recovery of Al and Cr from BCR-CRM 279 sea lettuce
| Metal | Spiked mass (μg) | Recovery (%) (n = 3) |
| Al | 100 | 95 ± 3.5 |
| Cr | 10 | 102 ± 4 |
4 Conclusion
The application of the Plackett–Burman designs, which are regrettably frequently ignored by the analysts, is a rapid, economical and efficient way of knowing the main factors influencing the analysis quality. In the present article, only 28 experiments were carried out to evaluate the influence of 25 factors on the determination of Pb, Cr and Al in macroalgal samples. In the chosen experimental field, it is recommended to add small volumes of HCl and HF acids (3 and 5 ml, respectively). The factors considered not the most influent can be maintained at the high level (+) or the low level (−) according to the analyst requirements.


