1 Introduction
The structures of hydroxy anions, present in single crystals and not acting as ligands or bridges to metals have, mistakenly, been thought as being relatively simple species acting primarily as charge compensating entities. Hereby, we put this idea to rest. Some, as in the case of H3O2−, are found as bridging ligands to metal complexes such as is the case with compounds described in a review by Ardon and Bino [3]. Here, we describe hydrated hydroxy anions which are not acting as ligands to metals and are embedded in crystalline lattices in the form of [(H2O)n(OH)]−. Efforts at understanding the optimized geometries and energetics of those with n = 1–3 has been published [4]. Additional theoretical papers were published by Haymet and associates [5,6].
Our search of the CSD produced 10 papers describing the crystallography of crystals containing hydrated hydroxide anions which met the following criteria:
- (a) Using the search engine ConQuest (version 1.7) of CSD1 the search was carried out with the graphic motif “OH−”. Since no further compositional conditions were imposed on the search, the entire data base was searched, provided that (1) there was minor or no disorder in the hydronium ion itself; however, if there is disorder in a charge compensating ion, solvent molecule of crystallization or a molecule of a co-crystal, the example may have been used in this review provided that the hydrated anion had sensible stereochemistry. If the same anion was found in an ordered crystal, the disordered structure which was ignored, at least for the graphics; though, it might have been mentioned in the text. (2) If the space group was corrected in a subsequent publication, and accepted by CSD, the latest entry was used. (3) Finally, no powder diffraction data were accepted since they would never produce accurate positions of hydrogen atoms, if at all. (4) A separate search was carried out using the combined motifs (OH− + neutron diffraction) and no further restrictions. Ten structures were listed in the search results. All of them were structures of organic compounds (e.g. 1,2-benzene dicarboxylic acid) whose acidic hydrogens were hydrogen bonded to a hydroxide anion. None of them were hydrated clusters; therefore, they did not qualify for inclusion in this review. (5) Since the structures accepted for this review were all the result of X-ray diffraction, the question of precision and accuracy of the hydrogen positions may be questioned by purists.
- (b) Individually, every entry found was examined as a diagram and as a 3-D figure using the Mercury routine of ConQuest. If the 3-D picture showed all the expected hydrogens, given the chemical formulation, the entry was saved. If missing or obviously distorted, the entry was rejected.
The resulting data set, deemed acceptable, was saved and used for a more detailed analysis using the packing diagram routine of DIAMOND [2]. The results are classified into the following categories: (a) acyclic systems (these are further subdivided by the number of oxygens in the anion and being discrete or condensed). If there are geometrical isomers or conformers, these were graphically illustrated. (b) Cyclic systems, classified by ring size. Here, isolated and condensed systems will also be dealt with separately. (c) Highly condensed systems will be dealt separately, and last.
It has been assumed that if the structural data appeared in the CSD compendium, and passed all the additional test we imposed, that there are no seriously faulty structures in this review, unless they were identified as being so. One of the problems with these analyses of structural data is that recognition of the correct structure is often obscured by being oriented in arbitrary crystallographic directions that require a lot of persistent work to document properly. A strenuous effort was made to ensure that no mistakes were made by selecting a line of sight which was not the true and unique one.
Note that, as in the case of waters of crystallization, hydrated hydroxide anions are anchored to the parent species of the lattice by (a) hydrogen bonds to suitable Lewis bases, such as the oxygens of CO, NO, NO2, carboxylates, –NH2 functional groups or (b) to Lewis acids such as carboxylate hydrogens, in which case the oxygen of the water or hydronium ion is the Lewis base donor. It is because of this feature that more complex species, such as H5O3− (and larger anions) are either symmetrical or asymmetrical. In the former case, hydrogen bonding is equally strong on all directions. In the asymmetrical cases, the hydrogen bonding to one end is stronger than the species expanding in the direction of the stronger tug.
The figures are color coded. That is, symmetry related bonds are all of the same color. In that way, only one bond distance is necessary and clutter is avoided, particularly in the case of small, or fused rings. When, accidentally, two bonds are of the same length (within experimental error) but are not symmetry related, different colors were used. This occurred very rarely; nonetheless, it was observed.
Finally, only 10 species were kept in the graphics file presented here. Because the stereochemistry of some of the anions is quite complex (some are infinite in one or more directions) we had to resort, in some cases, to use more than one figure. The additional figures describe smaller, recognizable, pieces of the larger ones. This was certainly the case with SEYLAJ which is a highly condensed set of three, four- and five-membered, fused, rings. All figures show (upper left corner) the direction along which the picture is oriented with respect to the crystallographic axes.
2 Finite acyclic systems
DAPSCR10 [7] – The anion contains two oxygens, its overall composition is H3O2−. The water is the Lewis acid, donating one of its hydrogens to a non-bonded pair of the Lewis base. See Fig. 1. In this Cr(III) coordination compound the ligand has a dangling –NH2 moiety which is hydrogen bonded to a water (note this distinction for comparison with EXOQIR and with LICQIX).
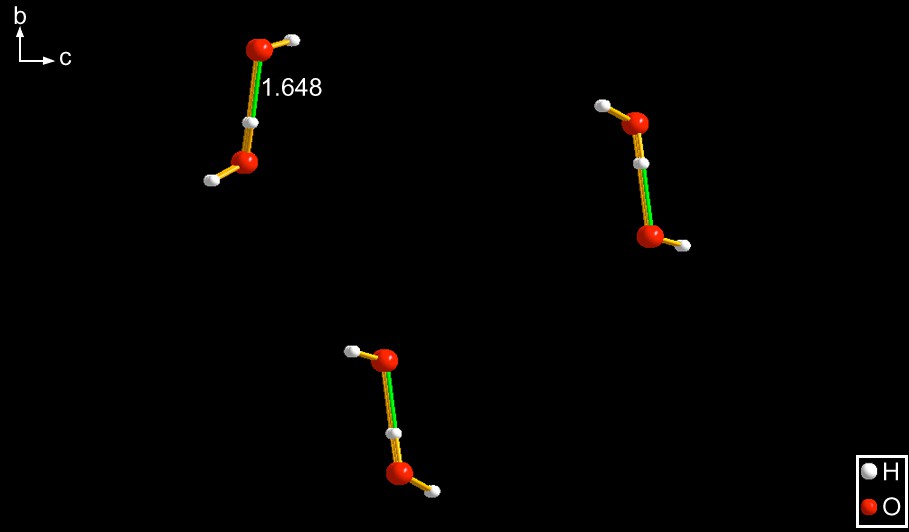
The geometry of DAPSCR10 [7]. Note the length of the hydrogen bond (1.648 Å) and compare it with EXOQIR whose geometry is quite similar. This is one of those cases referred to in Section 1 in which the hydrogen bond length changes due to differences in the bonding strength of the two ends with their anchoring points in their respective lattices.
EXOQIR [8] – Also consisting of two oxygens and overall composition H3O2−. In this case, also, the water is the Lewis acid, donating one of its hydrogens to a non-bonded pair of the Lewis base. However, the hydrogen bond is much weaker, having a length of 1.828 Å. See Fig. 2.
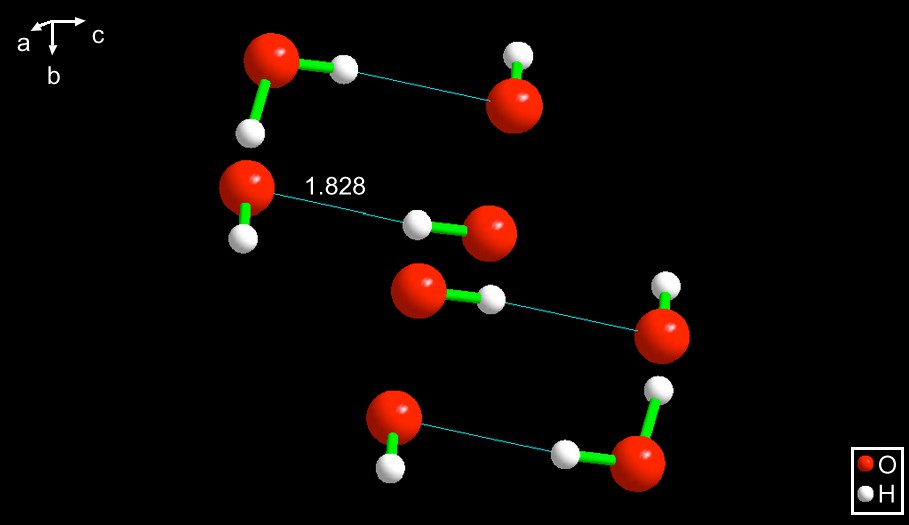
EXOQIR [8]. Note that in this case, as well as in the case of DAPSCR10, the hydrogens at the end of the anion (not participating in its hydrogen bond) are trans to each other. Interestingly, the hydroxide anion is hydrogen bonded to a hydrogen of an –NH3 ligand to the central iron by a bond of ca. 2.0 Å.
LICQIX [9] – The composition of this species is the same as the previous two cited above; namely, H3O2−. However, in this anion the hydroxide is the Lewis acid, being the proton donor to the water. Interestingly, the hydrogen bond length is 1.605 Å, which is a little shorter than that in DAPSCR10. See Fig. 3.
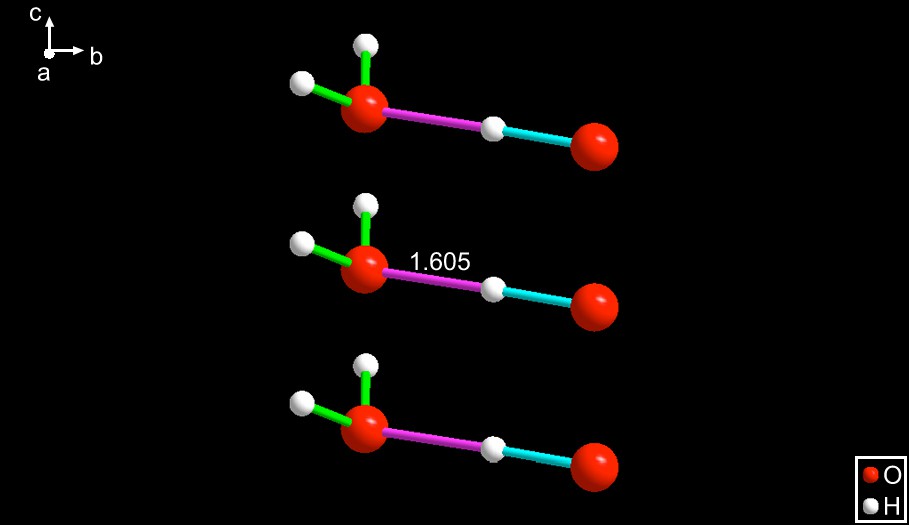
LICQIX [9]. This is a structural isomer of DAPSCR and EXOQIR (compare this figure with Figs. 1 and 2). The cause of this reversal of function is not clear since, as in the case of EXOQIR, a non-bonded pair of the hydroxide forms a substantial hydrogen bond with a hydrogen of a quaternary (S–NH2–C) of the ligand binding a dinuclear Ni(II) complex. Those NH2 hydrogens must be partially positively charged, being connected to a quaternary nitrogen; however, the role reversal observed in this species may not be associated with this hydrogen bond to a metal ligand since it should have occurred in the case of EXOQIR as well, and it does not.
VORYEG [10] – This is a Rh organometallic compound crystallizing in the space group R(). The metal sits at a () axis and is surrounded by a hydrated hydroxide anion shown in Figs. 4 and 5.
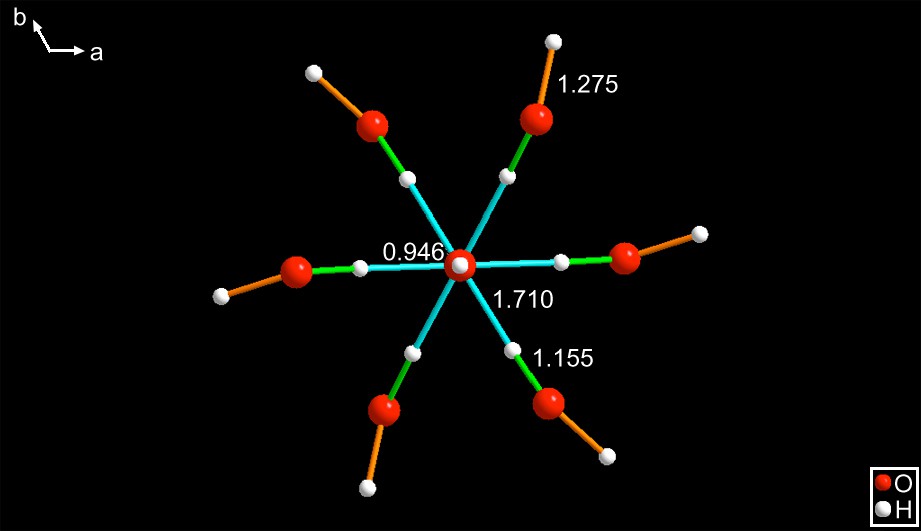
The anions shown in a c-axis projection. It has a central hydroxide whose hydrogen is aligned with the c-axis and has an O–H distance of 0.946 Å. The figure is deceptive because it appears to be a star-shaped molecule with six arms. That is because this figure is showing a pair of adjacent layers related by the (−3) roto-inversion operator. When viewed along an arbitrary direction, the correct representation differs, see Fig. 5.
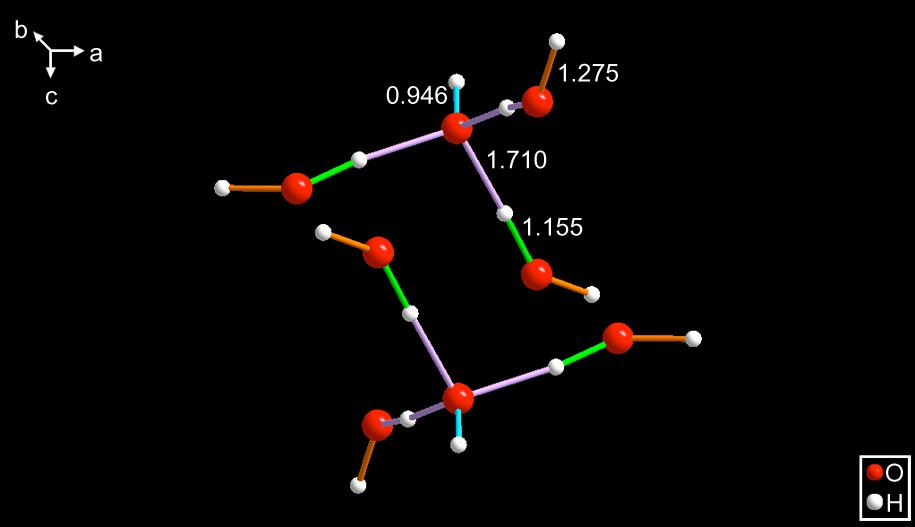
Now that view is approximately along the (111) direction, one can see the relationship between adjacent pairs of anions and the hydrogen atom belonging to the hydroxide, whose geometry is, approximately, tetrahedral. The previously discussed species contained three-bonded hydroxides. This one is four-bonded.
3 Infinite acyclic species
PAZDOJ [11] – This anion is an infinite, 1-D, string consisting of a repetition of the motif of (⋯OH–H2O⋯)n extending along the a-axis of the orthorhombic space group Pnma. Each hydroxide anion is flanked by two hydrogen bonds from two adjacent waters and due to the geometry of the non-bonded pairs of the hydroxide oxygen, the structure around oxygen is trigonal. Therefore the overall anion is slightly modulated. See Fig. 6.
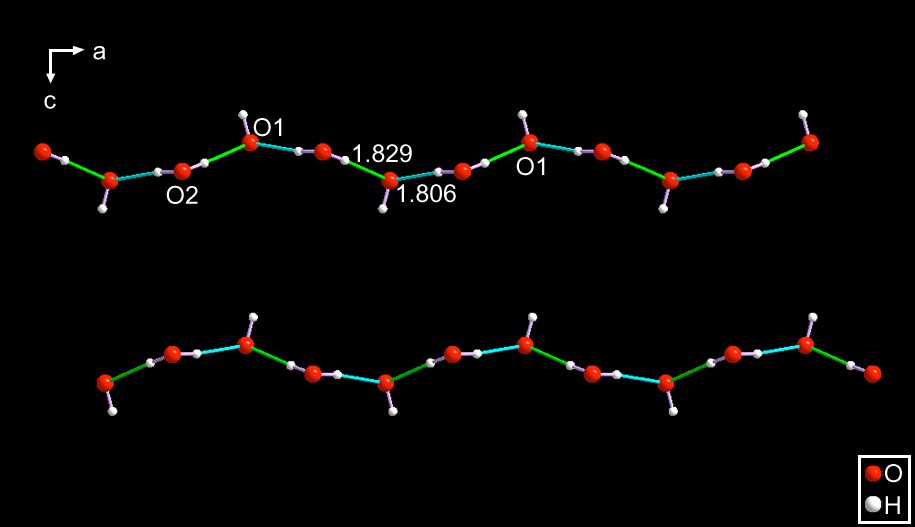
PAZDOJ [11]. Since these figures are all color coded, it is evident that there are only two independent bonds around the hydroxide oxygen (O1). The hydrogen bonds are substantial (ca. 1.829 and 1.806 Å). The space group is Pnma and the hydrated hydroxide anionic strings extend along the a-axis.
SEYLEN012 – Just as in the case of PAZDOJ [11] the anion consists of an infinite chain with a repeat unit of (⋯OH–H2O⋯)n In fact, they are identical with those present in PAZDOJ. The major difference between the anions is the fact that in this one the two hydrogen bond distances are 1.855 and 2.000 Å. Again, this is a case of the influence of the anchoring points to which the anions are bound to. See Fig. 7.
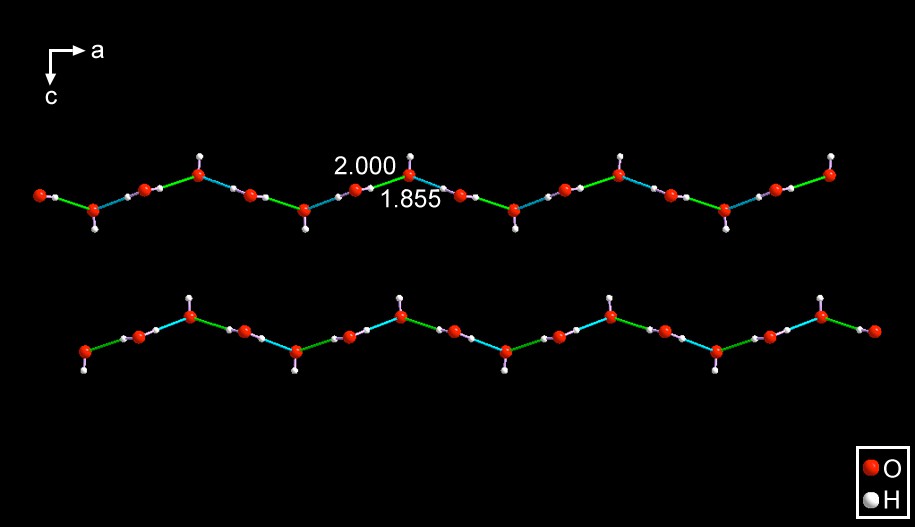
It is interesting to note that PAZDOJ and this compound are, both, hydrated crystals of alkyl ammoniun hydroxides. However, in the former the ammonium cation is a five-membered, aliphatic ring; SEYLEN01 has an acyclic ammonium cation. Yet, they both crystallize in Pnma and the anionic strings extend along the a-axis.
4 Monocyclic species
We found none in the survey of structures which fit the conditions outlined in Section 1. If there are some species in this category, they have either not been studied, so far, or they had hydrogen atoms missing or disordered.
5 Cyclic, fused species
MATLEY [12] – The four-membered rings present in this ribbon consist of three waters and one hydroxide, the latter being identified by having purple and green hydrogen bonds of 1.818 and 1.878 Å. The rings are fused at corners which are occupied by waters. See Fig. 8.
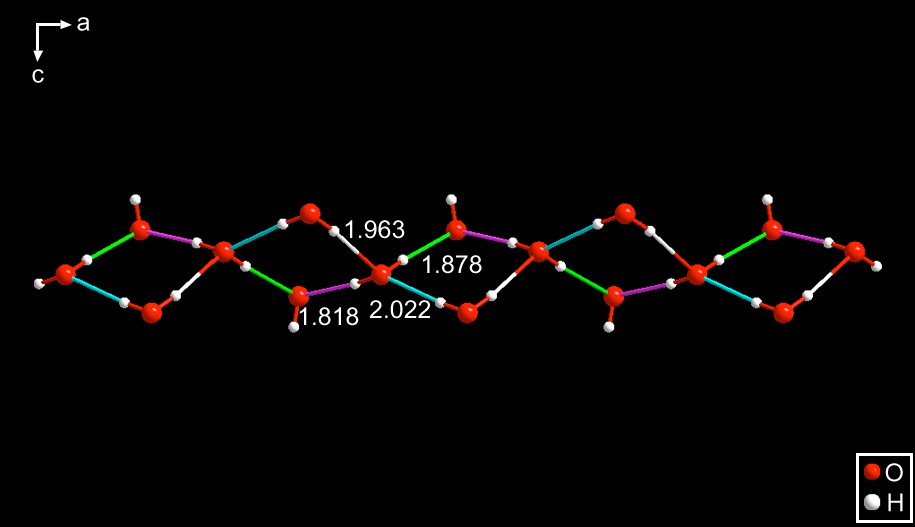
This is a hydroxide derivative of the tetraethyl ammonium cation. Interestingly, as was the case for PAZDOJ and SEYLEN01, it crystallizes in space group Pnma, but with a totally different motif for the anion which is a somewhat puckered species with four, somewhat different, hydrogen bond lengths. Note that the hydroxide alternates in position in the chain, the left-most being at the top of the ring.
QIJDES [13] – The anion present in this substance consists of two fused three-membered rings sharing a face. One of the edges of that array shares a face with a four-membered ring of waters. See Fig. 9.
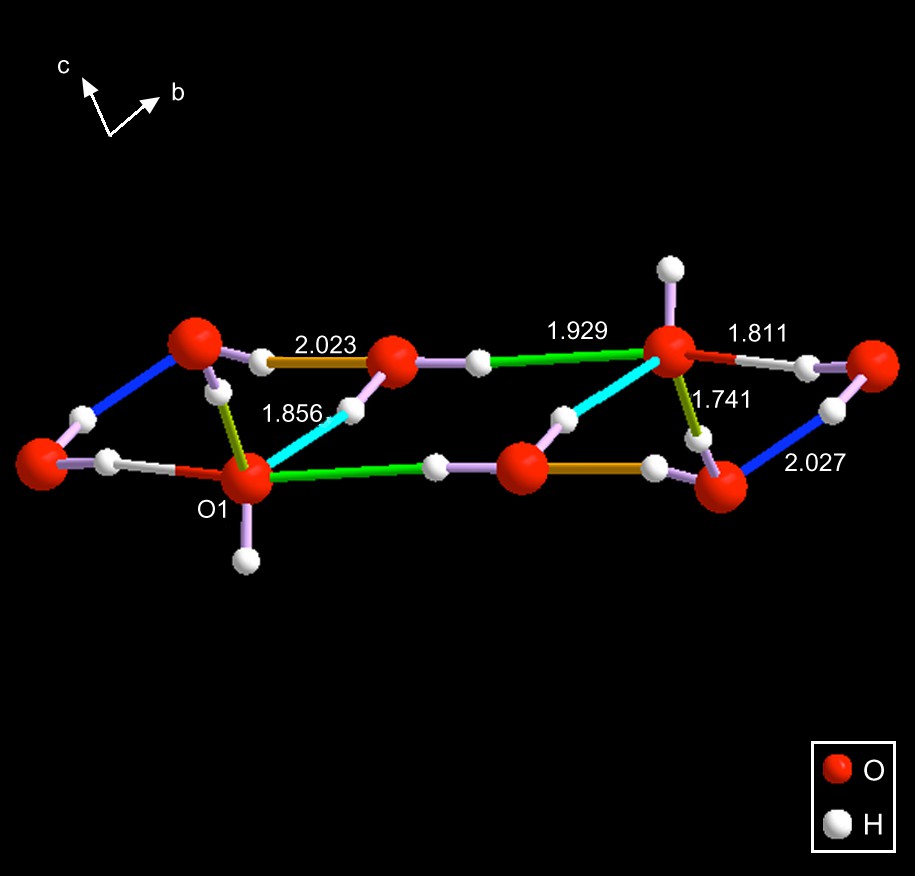
The hydroxide oxygen is labeled O1. Interestingly, it is bonded to five hydrogens: its own, and four additional hydrogen bonds (to waters) having the lengths given in the figure. These bonds are substantially below the usual limit of 3.25 Å; in fact, the longest is barely more than 2.0 Å. Similar five-bonded oxygens were observed in the case of the hydronium cation known in CSD as OBATAM.3
SEYLAJ [14] – This amazing compound is exactly the same tetramethyl ammonium species as SEYLEN01; however, the anions are totally different because they have different amounts of water of hydration. SEYLEN01 has one water and one hydroxide; the current compound has four waters. Therefore, they constitute an interesting, and different, case of hydration polymorphism in which the stereochemistry of the anions is totally different. See Figs. 10–12.
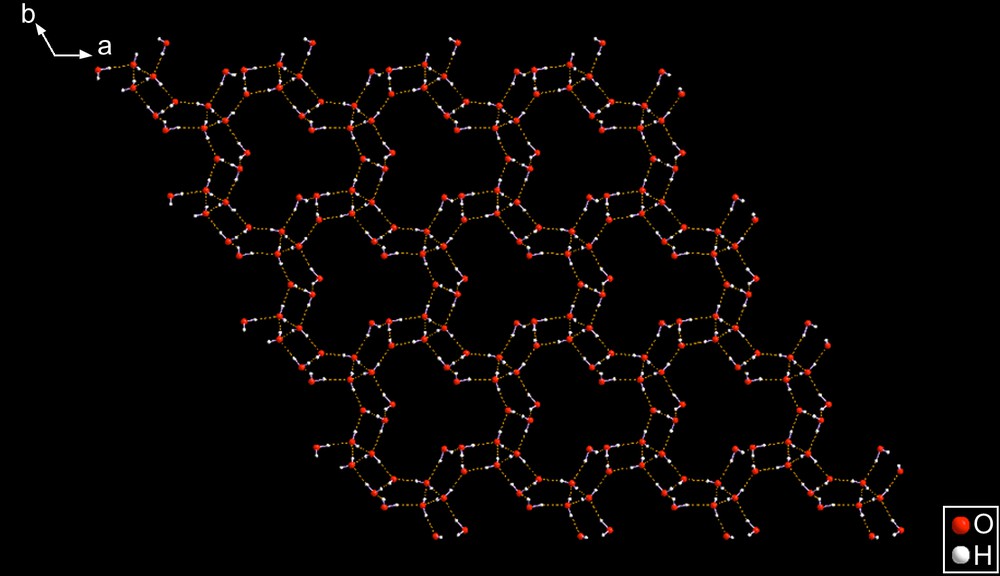
SEYLAJ crystallizes in the space group P31, SEYLEN01 in Pnma. The anion is an infinite collection of fused rings having three-fold internal geometry since their center is a three-fold axis. The entire sheet is centred at a three-fold axis, as well. Smaller sections are shown in Figs. 11 and 12 which permit a more detailed perusal of the complexity of this gigantic species.
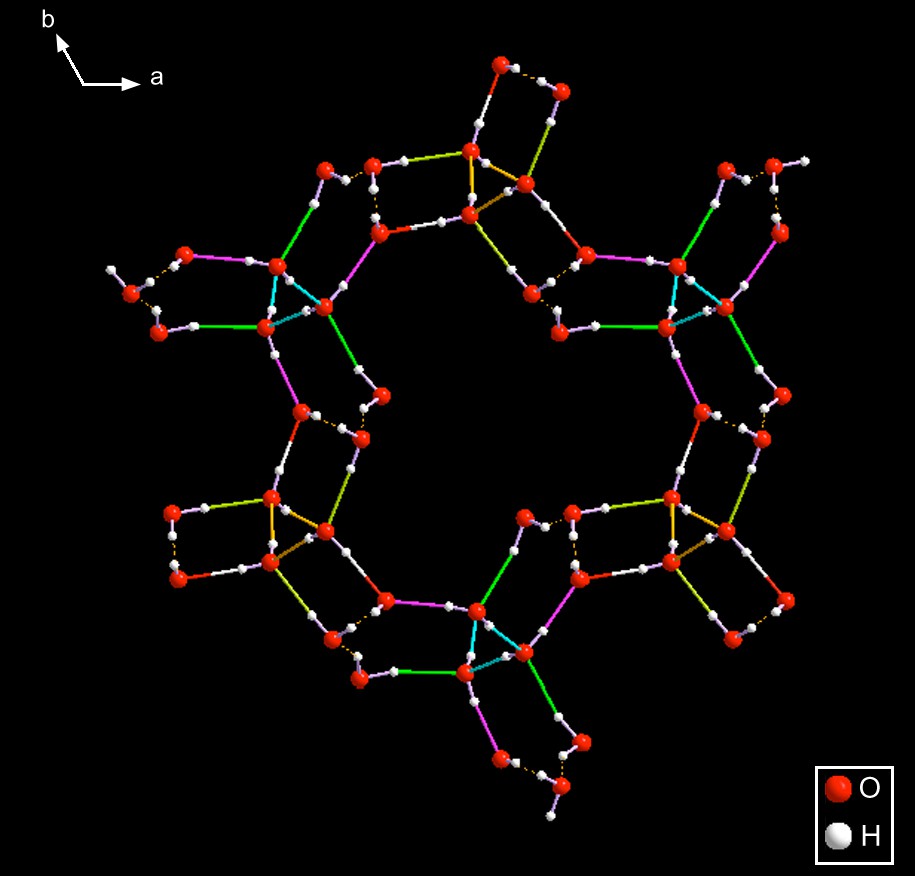
One of the component sub-rings constituting the totality of SEYLAJ. Here, one can appreciate the fact that there are even smaller motifs that are repeated so as to constitute the entire 2-D sheet that is present in this lattice. For a more detailed, and enlarged, figure of the smallest repeating fragment for the entire pattern, see Fig. 12.
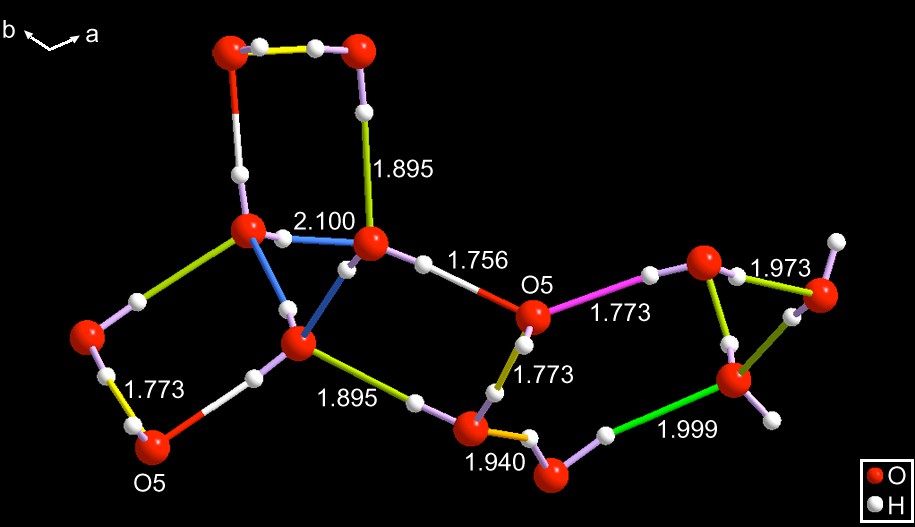
SEYLAJ has two distinct components: (a) there are two central, three-fold rings sitting at a three-fold symmetry sites and they are of slightly different sizes. (b) One has fused to it three four-membered rings related by the trigonal axis. The other one has three fused five-membered rings. (c) A corner oxygen (having one purple and one two-color bonds) is shared by one four and one five-membered rings. That oxygen is labeled O5. The final set of hydrogen bonds are given in this figure.
WOBSAH [15] – The anionic motif is an infinite 2-D sheet consisting of fused rings of four and six oxygens. The pattern shown here can be described as 2-D ribbons running approximately along the a-axis which are attached to adjacent sheets by hydrogen bonds. See Fig. 13.
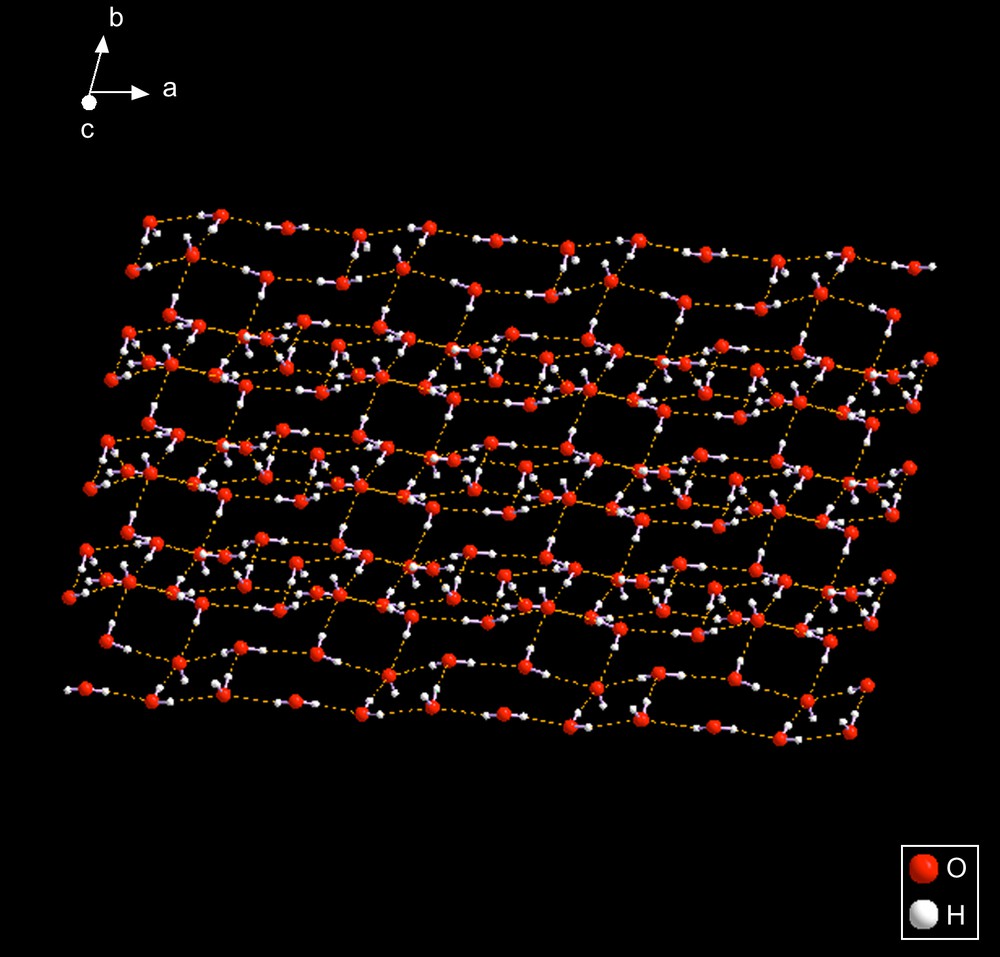
WOBSAH. This figure shows the complexity of the packing of waters and hydroxides. The entire pattern is 3-D but it is too complex to color code the bonds. A smaller, but representative section, is shown in Fig. 14. The pattern is oriented along the a-axis, as shown by the display of the axes (upper left corner).
6 Conclusions
- (1) As was the case with the hydronium cations [1] the hydronium anions occur in easily recognized patters; namely, acyclic and cyclic species. Also, the species can be finite or infinite in length. This distinction was made in the text.
- (2) The acyclic can be categorized as being linear or branched. In either case, the hydroxide moiety can act as a base to water and accept a proton or vice versa. For example, Figs. 1 and 2 display anions in which the hydroxide is the base, whereas in Fig. 3 the hydroxide is the acid. See text for more details.
- (3) The cyclic species can be separated into simple or fused. The latter can be finite or infinite. These categories have been illustrated in the text. Among the infinite ones, MATLEY (Fig. 8) is specially interesting because it is very similar to the hydronium cation OBATAM3, shown below.
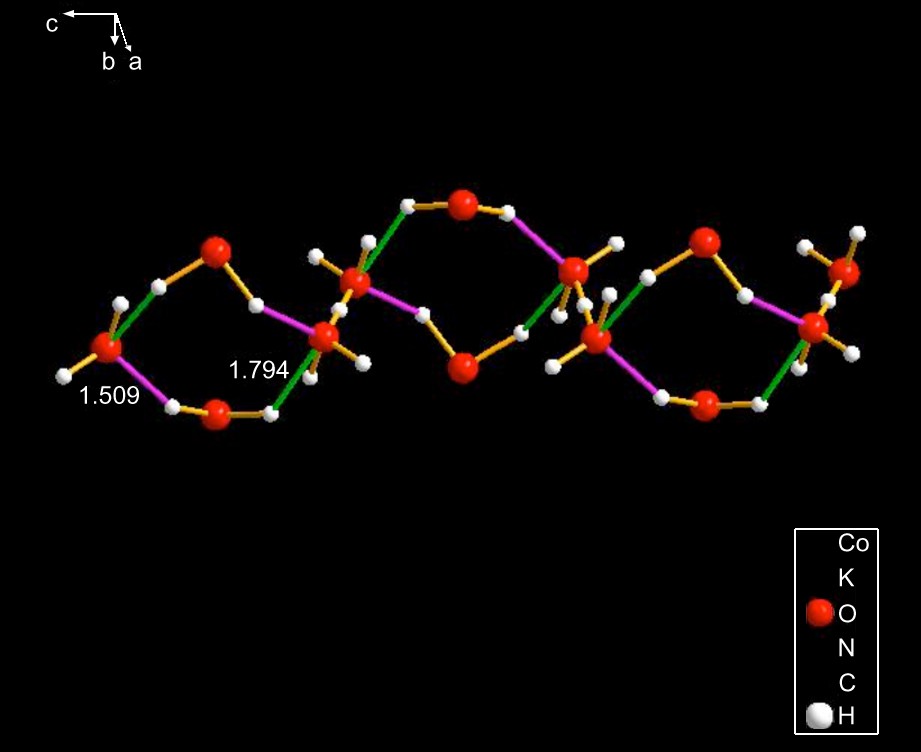
In OBATAM, an H3O+ species provides the hydrogen bonding linking the four-membered rings, whereas in MATLEY (Fig. 8) the link is provided by a water.
1 CSD (Cambridge Structural Database), Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK, Tel.: +44 1223 336408. Available from <http://wwsw.ccdc.cam.ac.uk>. Released by Wavefunction, Inc. 18401 von Karman Ave., Suite 370, Irvine, CA 92612. Tel.: +1 949 955 2120. Fax: +1 949 955 2118. <http://www.wavefun.com>. Release of April 2005. The most recent version of CSD (1.8) was distributed by the Cambridge Crystallographic Data Centre (2006).
2 SEYLEN01 – See Ref. [6]; however, the former crystallizes as a trigonal crystal (P31), this one is orthorhombic (Pnma). The packing motifs are totally different – the former is cyclic, the latter is acyclic.
3 OBATAM is discussed in a review of hydronium cations by Ivan Bernal, The Composition, Charge and Architecture of Hydronium Ions as Observed in the Crystalline State, Comptes Rendus, French Academy of Sciences, see Ref. [1].


