1 Introduction
2,3-Dihydroquinazolines, an important class of heterocyclic compounds, exhibit a wide spectrum of biological activities and pharmacological properties [1]. Additionally, these compounds can easily be oxidized to their quinazolin-4(3H)-ones analogues, which are themselves important biologically active compounds [2,3]. On the other hand, a large variety of substituted benzimidazole or benzothiazole groups heterocyclic compounds have been found to possess in vivo and in vitro growth-inhibiting activity against various strains of bacteria, fungi and viruses [4]. Because of this, numerous approaches for their synthesis have been reported in the literature [5].
Multi-component reactions (MCRs) have recently been discovered to be a powerful method for the synthesis of organic compounds, since the products are formed in a single step and diversity can be achieved by simply varying each compound [6]. During recent years, ionic liquids have attracted interest as environmentally benign reagents, due to their favorable properties, and a variety of catalytic reactions have been successful using ionic liquids [7]. The solvophobic properties of ionic liquids are able to generate an internal pressure and promote the association of the reactants in a solvent cavity during the activation process and accelerate a reaction. This property of ionic liquids is very efficient for MCRs, in which the entropy of reaction is decreased in the transition state.
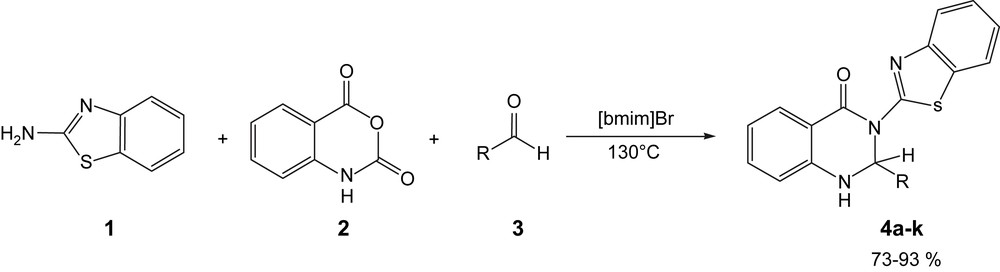
During the course of our studies on the synthesis of substituted benzimidazole or benzothiazole heterocyclic compounds [8] and our interest in multi-component reactions [9], we became interested in the possibility of synthesising benzimidazo-, triazolo-, or benzothiazo-quinazoline derivatives by the one-pot three-component condensation reactions strategy of 2-aminobenzimidazole or 2-aminobenzothiazole 1 or 3-amino-1,2,4-triazole with isatoic anhydride 2 and aldehyde 3 under classical heating conditions in 1-butyl-3-methylimidazolium bromide [bmim]Br as an ionic liquid (Scheme 1).
2 Results and discussion
This one-pot method involves the classical heating of a mixture of 2-aminobenzothiazole 1, isatoic anhydride 2 and aldehyde 3 without using any catalyst in ionic liquid to give a new family of 3-(2′-benzothiazolyl)-2,3-dihydroquinazolin-4(1H)-ones 4 in high yields.
In order to examine the best ionic liquid, various ionic liquids such as tetramethylguanidinium trifluoroacetate (TMGT), tetramethylguanidinium acetate (TMGA), tetrabutylammonium bromide (TBAB), tetrabutylammonium chloride (TBAC), methylimidazolium trifluoroacetate (MIT), 1-butyl-3-methylimidazolium bromide ([bmim]Br), 1-butyl-3-methylimidazolium hexafluoroposphate ([bmim]PF6) were applied in this condensation reaction (Table 1). In the course of this study, it was found that 1-butyl-3-methylimidazolium bromide was the best ionic liquid for this reaction in terms of yield and easy work-up. To illustrate the need for [bmim]Br in these reactions, the reaction of 2-aminobenzothiazole 1, isatoic anhydride 2 and aldehyde 3 was studied in the absence of [bmim]Br. The yield of product was trace at 130 °C after 30 min (Table 1, Entry 1).
One-pot synthesis of 3-(2′-benzothiazolyl)-2,3-dihydroquinazolin-4(1H)-one at different ionic liquid conditions at 130 °C
| Entry | Ionic liquid | Time (min) | Yield (%) |
| 1 | – | 30 | Trace |
| 2 | TMGT | 30 | 63 |
| 3 | TMGA | 30 | 27 |
| 4 | TBAB | 30 | 83 |
| 5 | TBAC | 30 | 79 |
| 6 | MIT | 30 | 64 |
| 7 | [bmim]Br | 30 | 90 |
| 8 | [bmim]PF6 | 30 | 74 |
In order to improve the yields, we performed reactions using different quantities of reagents. The best result was obtained with 1:1:1.1 ratios of 2-aminobenzothiazole, isatoic anhydride, and aldehyde in the presence of 0.3 g of [bmim]Br.
The efficiency of the reaction is mainly affected by the amount of [bmim]Br and temperature. The best results have been obtained at 130 °C with an amount of 0.3 g of [bmim]Br and the yield of reaction is not considerably enhanced by increasing the amount of [bmim]Br or temperature. It is important to note that in the absence of [bmim]Br, the reaction yield was trace even at 130 °C after 1 h.
To explore the scope and limitations of this reaction, we have extended it to aliphatic and various ortho-, meta- and para-substituted benzaldehydes in the presence of 2-aminobenzothiazole, 2-aminobenzimidazole and 3-amino-1,2,4-triazole. As indicated in Table 2, the reaction proceeds efficiently with benzaldehyde, electron-withdrawing and electron-releasing ortho-, meta- and para-substituted benzaldehydes and aliphatic aldehydes. However, the reaction with 3-amino-1,2,4-triazole and 2-aminobenzoimidazole is completely stopped after heating at 130 °C for 30 min. This is probably a result of the lesser nucleophilicity of the NH group of 3-amino-1,2,4-triazole and 2-aminobenzimidazole compared to 2-aminobenzothiazole.
One-pot synthesis of 3-(2′-benzothiazolyl)-2,3-dihydroquinazolin-4(1H)-one by the condensation reaction of 2-aminobenzothiazole, isatoic anhydride and aldehyde in ionic liquid at 130 °C
| Product | R | Time (min) | Yield (%) | Mp (°C) |
| 4a | 4CH3–C6H4– | 30 | 91 (90,90,89,89)a | 198–199 |
| 4b | C6H5– | 30 | 93 | 233–236 |
| 4c | 4CH3O–C6H4– | 30 | 92 | 184–186 |
| 4d | 4Cl–C6H4– | 30 | 84 | 190–193 |
| 4e | 4Br–C6H4– | 40 | 86 | 231–234 |
| 4f | 4NO2–C6H4– | 60 | 79 | 245–246 |
| 4g | 3Br–C6H4– | 30 | 84 | 183–186 |
| 4h | 3NO2–C6H4– | 30 | 81 | 251–253 |
| 4i | 2CH3–C6H4– | 30 | 85 | 198–200 |
| 4j | 2CH3O–C6H4– | 30 | 88 | 225–230 |
| 4k | CH3– | 60 | 73 | 215–222 |
| 4l | C6H13– | 50 | 75 | 148–151 |
a The same ionic liquid runs for four times.
The structure of the products 4 was deduced from their IR, 1H NMR, 13C NMR and MS spectra. The mass spectra of these compounds displayed molecular ion peaks at appropriate m/z values.
The 1H NMR spectrum of 4a in DMSO-d6 exhibited a sharp line readily recognized as arising from a methyl group (δ = 2.17 ppm) and a multiplet appeared at δ = 6.93–8.30 ppm for aromatic protons, and one doublet of doublet (at 7.08 and 7.17 ppm, J = 7.7 Hz) for (CH3C6H4), and a doublet for methine proton at 7.51 ppm, J = 3.01 Hz, and a doublet at 8.30 ppm, J = 3.20 Hz for the NH proton. The 1H spectra of 4b–k in DMSO-d6 are similar to those of 4a, except for the R group, which exhibits characteristic signals with appropriate chemical shifts.
The 1H decoupled 13C NMR spectrum of 4a showed 20 distinct resonances in agreement with the suggested structures. The 1H and 13C NMR spectra of 4b–k are similar to those of 4a except for the R1 and R2 groups, which exhibit characteristic signals with appropriate chemical shifts.
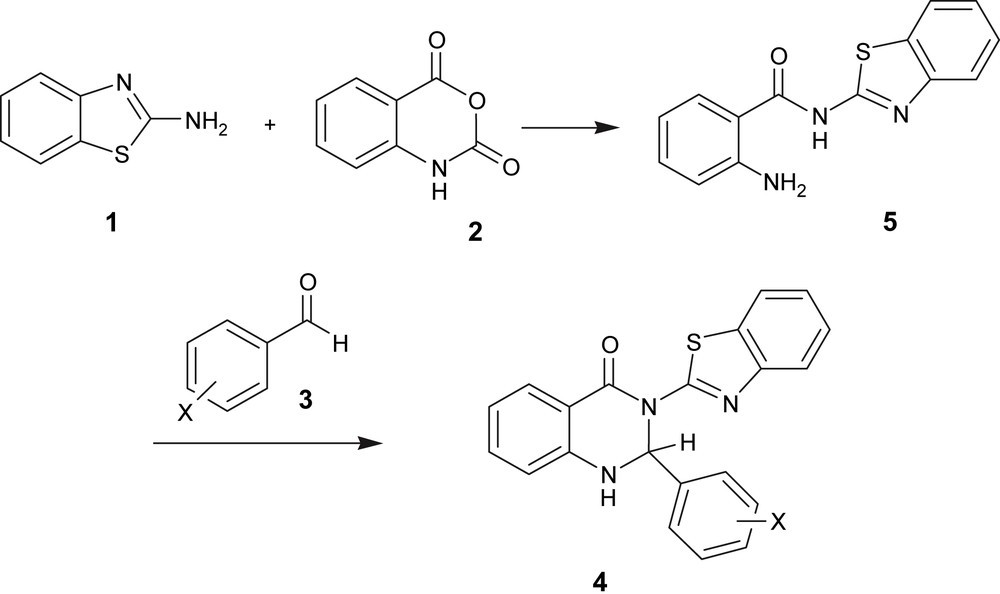
We have not established a mechanism for the formation of 2,3-dihydroquinazolin-4(1H)-ones ring systems, but a reasonable possibility is indicated in Scheme 2. The reaction presumably proceeds in two steps: condensation of 2-aminobenzothiazole 1 and isatoic anhydride 2, then aldehyde 3 is reacted with compound 5 through an imine synthesis and then cyclisation with addition of an amid nitrogen in the imine group.
One of the advantages of ionic liquids is their ability to be recyclable as reaction medium. We were able to separate [bmim]Br from the reaction medium easily by washing with water and evaporating the solvent under vacuum, and reuse it for subsequent reactions (Table 2, Entry 4a).
In summary, we have introduced an efficient and environmental friendly approach for the three-component condensation reaction of 2-aminobenzothiazole, isatoic anhydride and aldehyde for the synthesis of a new class of heterocyclic 2,3-dihydroquinazolin-4(1H)-ones ring systems in high yields, which the one-pot nature and solvent-free protocol in the absence of any catalyst make an interesting approach. To the best our knowledge, this is the first report of a synthesis of 2,3-dihydroquinazolin-4(1H)-ones in an ionic liquid, and these new reaction conditions open an important alternative to the use of toxic solvents.
3 Experimental
3.1 General methods
Melting points were measured on the Electrothermal 9100 apparatus and are uncorrected. IR spectra were measured on a Bomen FT-IR-MB 100 spectrometer. 1H and 13C NMR spectra were measured with a Bruker DRX-300 Avance spectrometer at 300 and 75 MHz. Chemical shifts (δ) are reported in ppm and coupling constants (J) are reported in hertz (Hz). Mass spectra were recorded on a Shimadzu QP 1100 EX mass spectrometer. Chemicals were obtained from Merck and Fluka and used without further purification.
3.2 Typical procedure for the synthesis of 3-(2′-benzothiazolyl)-2,3-dihydro-2-(4-methylphenyl)-4(1H)-quinazolinone (4a)
A mixture of isatoic anhydride (0.130 g, 1 mmol), p-methylbenzaldehyde (0.132 g, 1.1 mmol), and 2-aminobenzothiazole (0.150 g, 1 mmol) was successively added to a screw-capped vial containing a magnetic stirring bar, and the ionic liquid (0.3 g) was heated at 130 °C in a preheated oil bath for 30 min. Then the reaction mixture was washed with cold water (2 × 10 ml) and the solid residue was crystallized from ethanol to yield 0.23 g of 4a as colorless crystals (91%).
All the products are new compounds, which were characterized by IR, 1H NMR, 13C NMR, and mass spectral data.
3.2.1 3-(2′-Benzothiazolyl)-2,3-dihydro-2-(4-methylphenyl)-quinazolin-4(1H)-one (4a)
Colorless crystals; mp 198--199 °C; IR (KBr) (υmax, cm−1): 3340, 1631, 1609, 1506, 1432, 1254; 1H NMR (300 MHz, DMSO-d6): δH 2.17 (3H, s, CH3), 6.78 (1H, t, J = 7.29 Hz, CH), 6.93 (1H, d, J = 8.09 Hz, CH), 7.08 (2H, d, J = 7.68 Hz, 2CH), 7.17 (2H, d, J = 7.77 Hz, 2CH), 7.35 (1H, t, J = 7.80 Hz, CH), 7.38 (1H, t, J = 10.16 Hz, CH), 7.45 (1H, t, J = 7.25 Hz, CH), 7.51 (1H, d, J = 3.01 Hz, CH(sp3)), 7.77 (1H, t, J = 7.03 Hz, CH), 7.79 (1H, d, J = 6.95 Hz, CH), 8.04 (1H, d, J = 7.72 Hz, CH), 8.30 (1H, d, J = 3.20 Hz, NH); 13C NMR (75 MHz, DMSO-d6): δC 20.97, 68.36, 114.09, 116.23, 118.82, 121.37, 122.21, 124.56, 126.16, 126.75, 128.84, 129.54, 133.06, 135.93, 137.02, 138.02, 147.40, 148.14, 151.18, 162.13; MS (EI, 70 eV) (m/z, %): 372 (M + 2, 90), 237 (100), 165 (20), 130 (20), 77 (25).
3.2.2 3-(2′-Benzothiazolyl)-2,3-dihydro-2-(phenyl)-quinazolin-4(1H)-one (4b)
Colorless crystals; mp 233–236 °C; IR (KBr) (υmax, cm−1): 3350, 1630, 1612, 1501, 1431, 1389, 1228; 1H NMR (300 MHz, DMSO-d6): δH 6.79 (1H, t, J = 7.43 Hz, CH), 6.97 (1H, d, J = 8.12 Hz, CH), 7.22–7.40 (5H, m, C6H5), 7.37 (1H, t, J = 7.03 Hz, CH), 7.42 (1H, t, J = 7.44 Hz, CH), 7.44 (1H, t, J = 7.28 Hz, CH), 7.60 (1H, d, J = 3.59 Hz, CH(sp3)), 7.79 (1H, d, J = 8.13 Hz, CH), 7.82 (1H, t, J = 8.76 Hz, CH), 8.05 (1H, d, J = 7.72 Hz, CH), 8.38 (1H, d, J = 3.63 Hz, NH); 13C NMR (75 MHz, DMSO-d6): δC 68.45, 114.09, 116.26, 118.89, 121.40, 122.22, 124.58, 126.25, 126.76, 128.67, 128.91, 129.04, 133.11, 135.97, 140.04, 147.38, 148.15, 158.24, 162.13; MS (EI, 70 eV) (m/z, %): 223 (M-134, 75), 208 (100), 194 (20), 149 (50), 105 (30), 77 (50).
3.2.3 3-(2′-Benzothiazolyl)-2,3-dihydro-2-(4-methoxyphenyl)-quinazolin-4(1H)-one (4c)
Colorless crystals; mp 184–186 °C; IR (KBr) (υmax, cm−1): 3350, 1633, 1608, 1505, 1430, 1304, 1233; 1H NMR (300 MHz, DMSO-d6): δH 3.63 (3H, s, OCH3), 6.78 (1H, t, J = 7.34 Hz, CH), 6.83 (2H, d, J = 7.67 Hz, 2CH), 6.94 (1H, d, J = 8.08 Hz, CH), 7.21 (2H, d, J = 7.81 Hz, 2CH), 7.34 (1H, t, J = 7.13 Hz, CH), 7.39 (1H, t, J = 7.12 Hz, CH), 7.44 (1H, t, J = 7.52 Hz, CH), 7.52 (1H, brs, CH(sp3)), 7.78 (1H, d, J = 7.65 Hz, CH), 7.81 (1H, d, J = 7.61 Hz, CH), 8.04 (1H, d, J = 7.73 Hz, CH), 8.32 (1H, br s, NH); 13C NMR (75 MHz, DMSO-d6): δC 55.48, 68.21, 114.06, 114.39, 116.24, 118.79, 121.38, 122.20, 124.55, 126.74, 127.50, 128.85, 131.84, 133.07, 135.94, 147.42, 148.16, 158.19, 159.52, 162.12; MS (EI, 70 eV) (m/z, %): 388 (M + 2, 50), 253 (100), 239 (90), 167 (20), 77 (10).
3.2.4 3-(2′-Benzothiazolyl)-2,3-dihydro-2-(4-cholorophenyl)-quinazolin-4(1H)-one (4d)
Colorless crystals; mp 190–193 °C; IR (KBr) (υmax, cm−1): 3335, 1634, 1609, 1506, 1403, 1254; 1H NMR (300 MHz, DMSO-d6): 6.81 (1H, t, J = 7.35 Hz, 1CH), 6.96 (1H, d, J = 8.20 Hz, 1CH), 7.30 (2H, d, J = 8.53 Hz, 2CH), 7.36 (1H, t, J = 8.04 Hz, 1CH), 7.38 (2H, d, J = 8.34 Hz, 2CH), 7.42 (1H, t, J = 6.82 Hz, 1CH), 7.46 (1H, t, J = 7.17 Hz, 1CH), 7.54 (1H, d, J = 3.99 Hz, 1CH(sp3)), 7.78 (1H, d, J = 8.00 Hz, 1CH), 7.79 (1H, d, J = 7.67 Hz, 1CH), 8.06 (1H, d, J = 7.68 Hz, 1CH), 8.34 (1H, d, J = 4.06 Hz, NH); 13C NMR (75 MHz, DMSO-d6): δC 64.46, 113.57, 115.77, 118.55, 120.88, 121.61, 124.08, 126.21, 127.62, 128.37, 128.51, 132.58, 132.94, 135.49, 138.53, 146.58, 147.58, 157.59, 161.40; MS (EI, 70 eV) (m/z, %): 271 (27), 257 (70), 242 (100), 178 (35), 152 (50), 130 (25), 108 (20).
3.2.5 3-(2′-Benzothiazolyl)-2,3-dihydro-2-(4-bromophenyl)-quinazolin-4(1H)-one (4e)
Colorless crystals; mp 231–234 °C; IR (KBr) (υmax, cm−1): 3350, 1630, 1612, 1501, 1431, 1389, 1228; 1H NMR (300 MHz, DMSO-d6): δH 6.81 (1H, t, J = 7.47 Hz, 1CH), 6.96 (1H, d, J = 8.18 Hz, 1CH), 7.25 (2H, d, J = 8.25 Hz, 2CH), 7.36 (1H, t, J = 7.41 Hz, 1CH), 7.41 (1H, t, J = 8.39 Hz, 1CH), 7.45 (1H, t, J = 7.71 Hz, 1CH), 7.51 (2H, d, J = 8.60 Hz, 2CH), 7.53 (1H, d, J = 4.20 Hz, 1CH(sp3)), 7.78 (1H, d, J = 7.64 Hz, 1CH), 7.80 (1H, d, J = 7.42 Hz, 1CH), 8.05 (1H, d, J = 7.79 Hz, 1CH), 8.34 (1H, d, J = 3.88 Hz, 1NH); 13C NMR (75 MHz, DMSO-d6): δC 68.45, 114.09, 116.26, 118.89, 121.40, 122.22, 124.58, 126.25, 126.76, 128.67, 128.91, 129.04, 133.11, 135.97, 140.04, 147.38, 148.15, 158.24, 162.13; MS (EI, 70 eV) (m/z, %): 223 (75), 208 (100), 194 (20), 149 (50), 105 (30), 77 (50).
3.2.6 3-(2′-Benzothiazolyl)-2,3-dihydro-2-(4-nitrophenyl)-quinazolin-4(1H)-one (4f)
Oranges crystals; mp 245–246 °C; IR (KBr) (υmax, cm−1): 3373, 1647, 1614, 1523; 1H NMR (300 MHz, DMSO-d6): δH 6.81 (1H, t, J = 7.28 Hz, 1CH), 7.00 (1H, d, J = 8.13 Hz, 1CH), 7.34 (1H, t, J = 7.21 Hz, 1CH), 7.41 (1H, t, J = 7.50 Hz, 1CH), 7.44 (1H, t, J = 7.53 Hz, 1CH), 7.57 (2H, d, J = 8.28 Hz, 2CH), 7.67 (1H, s, 1CH), 7.77 (1H, d, J = 8.16 Hz, 1CH), 7.81 (1H, d, J = 7.81 Hz, 1CH), 8.05 (1H, d, J = 7.89 Hz, 1CH), 8.15 (2H, d, J = 8.11 Hz, 2CH), 8.46 (1H, s, 1NH); 13C NMR (75 MHz, DMSO-d6): δC 66.78, 112.77, 115.18, 118.06, 120.19, 120.98, 123.04, 123.43, 125.52, 126.44, 127.72, 131.86, 134.89, 145.64, 146.17, 146.58, 146.77, 156.82, 160.50; MS (EI, 70 eV) (m/z, %): 403 (25), 268 (100), 207 (50), 178 (20), 105 (20), 77 (25).
3.2.7 3-(2′-Benzothiazolyl)-2,3-dihydro-2-(3-bromophenyl)-quinazolin-4(1H)-one (4g)
Colorless crystals; mp 183–186 °C; IR (KBr) (υmax, cm−1): 3385, 1644, 1609, 1499, 1435, 1380, 1231; 1H NMR (300 MHz, DMSO-d6): δH 6.85 (1H, t, J = 7.33 Hz, 1CH), 6.90 (1H, d, J = 8.25 Hz, 1CH), 7.08–7.44 (6H, m, 6CHarom), 7.68 (1H, s, 1CH), 7.69 (1H, t, J = 8.04 Hz, 1CH), 7.71 (1H, d, J = 4.36 Hz, 1CH), 7.93 (1H, d, J = 7.45 Hz, 1CH), 8.01 (1H, d, J = 7.65 Hz, 1CH), 8.06 (1H, d, J = 4.37 Hz, 1NH); 13C NMR (75 MHz, DMSO-d6): δC 69.09, 113.40, 116.59, 119.14, 121.55, 122.17, 122.35, 124.67, 126.00, 126.74, 128.53, 128.75, 131.03, 133.03, 134.18, 136.12, 138.72, 145.85, 147.89, 157.28, 162.21; MS (EI, 70 eV) (m/z, %): 434 (M + 2, 40), 434 (M + 4, 30), 303 (100), 301 (98), 288 (90), 286 (90), 152 (35), 77 (35).
3.2.8 3-(2′-Benzothiazolyl)-2,3-dihydro-2-(3-nitrophenyl)-quinazolin-4(1H)-one (4h)
Colorless crystals; mp 251–253 °C; IR (KBr) (υmax, cm−1): 3440, 1637, 1619, 1539, 1524, 1434, 1340; 1H NMR (300 MHz, DMSO-d6): δH 6.82 (1H, t, J = 7.55 Hz, 1CH), 7.01 (1H, d, J = 8.18 Hz, 1CH), 7.35–7.48 (3H, m, 3CH), 7.57–7.64 (2H, m, 2CH), 7.69 (1H, d, J = 4.23 Hz, 1CH(sp3)), 7.78 (1H, d, J = 9.18 Hz, 1CH), 7.81 (1H, d, J = 9.06 Hz, 1CH), 8.07 (1H, d, J = 8.05 Hz, 1CH), 8.11 (1H, d, J = 8.13 Hz, 1CH), 8.28 (1H, s, 1CH), 8.47 (1H, d, J = 3.95 Hz, 1NH); 13C NMR (75 MHz, DMSO-d6): δC 67.30, 113.46, 115.97, 118.89, 120.73, 120.98, 121.83, 123.35, 124.26, 126.35, 128.49, 130.32, 132.05, 132.61, 135.75, 141.89, 146.36, 147.52, 147.98, 157.57, 161.26; MS (EI, 70 eV) (m/z, %): 403 (M + 3, 30), 354 (30), 268 (100), 207 (50), 105 (20), 77 (20).
3.2.9 3-(2′-Benzothiazolyl)-2,3-dihydro-2-(2-methylphenyl)-quinazolin-4(1H)-one (4i)
Colorless crystals; mp 198–200 °C; IR (KBr) (υmax, cm−1): 3330, 1631, 1609, 1501, 1452, 1389, 1231; 1H NMR (300 MHz, DMSO-d6): δH 2.72 (3H, s, CH3), 6.81 (1H, d, J = 7.83 Hz, 1CH), 6.82 (1H, t, J = 7.58 Hz, 1CH), 6.96–7.44 (7H, m, 7CHarom), 7.59 (1H, d, J = 4.09 Hz, 1CH), 7.71 (1H, d, J = 7.96 Hz, 1CH), 7.91–8.01 (2H, m, 2CHarom), 7.95 (1H, d, J = 4.48 Hz, NH); 13C NMR (75 MHz, DMSO-d6): δC 19.67, 66.92, 113.39, 116.21, 118.70, 121.48, 122.12, 124.00, 124.55, 126.53, 126.68, 128.74, 128.88, 131.67, 133.00, 135.85, 136.00, 138.61, 146.28, 147.99, 157.48, 162.40; MS (EI, 70 eV) (m/z, %): 372 (M + 2, 90), 237 (100), 193 (30), 165 (20), 105 (20), 77 (30).
3.2.10 3-(2′-Benzothiazolyl)-2,3-dihydro-2-(2-methoxyphenyl)-quinazolin-4(1H)-one (4j)
Colorless crystals; mp 225–230 °C; IR (KBr) (υmax, cm−1): 3346, 1647, 1614, 1511, 1460, 1440; 1H NMR (300 MHz, DMSO-d6): δH 3.94 (3H, s, OCH3), 6.71–6.89 (4H, m, 4CH), 7.07 (1H, d, J = 8.13 Hz, 1CH), 7.19–7.43 (4H, m, 4CH), 7.67 (1H, d, J = 3.45 Hz, 1CH), 7.73 (1H, d, J = 7.86 Hz, 1CH), 7.77 (1H, d, J = 3.48 Hz, 1NH), 7.87 (1H, d, J = 7.77 Hz, 1CH), 8.01 (1H, d, J = 7.77 Hz, 1CH); 13C NMR (75 MHz, DMSO-d6): δC 56.25, 65.71, 112.14, 113.05, 116.01, 118.49, 120.35, 121.46, 122.11, 124.54, 125.50, 126.68, 127.15, 128.68, 130.29, 133.04, 135.85, 147.15, 148.01, 157.08, 157.56, 162.65; MS (EI, 70 eV) (m/z, %): 388 (60), 253 (100), 238 (50), 167 (20), 132 (25), 77 (20).
3.2.11 3-(2′-Benzothiazolyl)-2,3-dihydro-2-methyl-quinazolin-4(1H)-one (4k)
Colorless crystals; mp 215–222 °C; IR (KBr) (υmax, cm−1): 3307, 1645, 1614, 1508, 1440. 1H NMR (300 MHz, DMSO-d6): δH 1.62 (3H, d, J = 6.04 Hz, CH3), 4.80 (1H, brs, NH), 6.72 (1H, q, J = 5.89 Hz, 1CH), 6.80 (1H, d, J = 8.08 Hz, 1CH), 6.95 (1H, t, J = 7.48 Hz, 1CH), 7.31 (1H, t, J = 7.26 Hz, 1CH), 7.43 (1H, t, J = 8.20 Hz, 1CH), 7.45 (1H, t, J = 7.79 Hz, 1CH), 7.84 (1H, d, J = 7.82 Hz, 1CH), 7.58 (1H, d, J = 7.58 Hz, 1CH), 8.07 (1H, d, J = 7.80 Hz, 1CH). 13C NMR (75 MHz, DMSO-d6): δC 19.68, 65.12, 113.06, 116.23, 118.47, 121.35, 122.02, 124.37, 126.60, 128.89, 133.01, 135.83, 147.38, 148.28, 127.45, 161.35. MS (EI, 70 eV) (m/z, %): 296 (M, 40), 280 (100), 161 (25), 77 (10).
3.2.12 3-(2′-Benzothiazolyl)-2,3-dihydro-2-hexyl-quinazolin-4(1H)-one (4l)
Colorless crystals; mp 148–151 °C; IR (KBr) (υmax, cm−1): 3370, 2926, 1633, 1513, 1435. 1H NMR (300 MHz, DMSO-d6): δH 0.80 (3H, t, J = 6.83 Hz, CH3), 1.27–1.29 (6H, m, 3CH2), 1.31 (2H, m, CH2), 1.66 (1H, m, CH2), 1.89 (1H, m, CH2), 6.37 (1H, ddd, J = 7.53 Hz, J = 6.37 Hz, J = 3.70 Hz, CH), 6.81 (1H, t, J = 7.58 Hz, CH), 6.90 (1H, d, J = 8.12 Hz, CH), 7.34 (1H, t, J = 7.49 Hz, CH), 7.42 (1H, t, J = 7.91 Hz, CH), 7.46 (1H, t, J = 7.32 Hz, CH), 7.67 (1H, d, J = 3.60 Hz, NH), 7.81 (1H, d, J = 7.99 Hz, CH), 7.82 (1H, d, J = 7.61 Hz, CH), 8.00 (1H, d, J = 7.78 Hz, CH). 13C NMR (75 MHz, DMSO-d6): δC 14.35, 22.44, 24.97, 28.40, 31.48, 32.48, 68.06, 113.49, 116.06, 118.35, 121.27, 122.05, 124.35, 126.62, 128.86, 132.98, 135.83, 147.18, 148.22, 157.68, 161.53. MS (EI, 70 eV) (m/z, %): 380 (M + 16, 25), 280 (100), 245 (30).
Acknowledgments
We gratefully acknowledge financial support from the Research Council of Shahid Beheshti University.


