1 Introduction
In 2005, a report appeared [1] which described the discovery of a rather unexpected species, namely, a cyclic hydronium cation of composition and charge H14O6+2. At the time, neither the existence of cyclic cations nor the fact that they could bear charges other than +1 was expected or suspected.
In 2006, a review was published [2] describing the stereochemistry of all the hydronium cations present in the Cambridge Structural Database (CSD).1 These were divided into acyclic and cyclic examples and, surprisingly enough, many were buried in that base but not noted by the authors of those studies, probably because there was no suspicion they could exist. Moreover, the graphics available in most cases were fairly clumsy. It requires, even now, a good deal of determination and hard work to discern their existence in crystal lattices, because, most of the time, the complete cluster is evident only by looking at the 3-D lattices in fairly arbitrary directions – as opposed to simple (001), (010), etc. projections.
In 2007, a report was published [3] announcing the discovery of a species with composition and charge of H18O8+2. In the discussions in Refs. [2,3] the suggestion was made that all the esoteric species newly described were the result of Nature acting as a skillful crystal engineer, providing clever traps to stabilize otherwise unstable species. Moreover, it was remarked [2,3] that the solution concentrations of something such as H14O6+2 or H18O8+2 must be incredibly low. Therefore, the traps must be extremely efficient in order to filter out such minute quantities of those hydronium cations.
In order to test the theory of stabilization, of unstable hydronium cations, by crystal engineering traps cleverly devised by Nature, we decided to study the stability of these species by three different types of theoretical calculations, described in Ref. [4]. These calculations were carried out using two different criteria
- (a) using the crystallographic coordinates found experimentally, what is the energy of a given molecule? As observed, are they stable or not?
- (b) allow the geometry of the molecule to vary so as to minimize energy. Is the resulting species stable or not?
- (c) given the fact that we found two totally different geometrical isomers of the same composition, namely, H14O6+2, is it possible to predict which is the more stable of the two? For details, the reader is referred to the paper in PNAS [4]. Briefly, those examined are stable either in the crystalline lattices where they were trapped, as well as isolated in a vacuum. (See Ref. [2] p. 1460 for discussion.)
Given this background, it seemed desirable to continue a search for additional examples of hydronium cations, which may be of novel new classes. That is what this report deals with.
2 The search for new hydronium cations
The search was carried out in October of 2007, using the current release of CSD,1 but limited to the years 2000–2006. There were 19 entries in the search, of which most were defective (see criteria for accepting an entry in Ref. [2]) or were known specimens of hydronium cations (see Fig. 1 and Ref. [5] where JEDYAT2 is displayed). JEDYAT is hydroxonium bis(N-(4-methyl-2-pyridyl)aminomethane-1,1-diphosphonic acid) chloride trihydrate, [(C7H12N2O6P2)2(H3O+)]Cl·3(H2O). Note that the authors describe the hydronium cation as having composition H3O+.
3 New hydronium cations
The first new species discovered is BEXFEQ [6], which is shown in Fig. 2. BEXFEQ is dicesium hydroxonium bis(μ2-1,3-diamino-2-propanolato-N,N,N′,N′-tetraacetato)-di-zirconium(IV)chloride tetrahydrate, (Cs+)2(H5O2+)[C22H26N4O18Zr2]2Cl·4(H2O). Note that the hydronium cation is formulated as H5O2+.

BEXFEQ is an acyclic cation of composition H11O5+. O22 is the H3O+ fragment of the cation, which is highly asymmetric, having one hydrogen-bonded water at one end (H3O+ is the proton donor on both sides of the chain). At the other end is a fragment of three hydrogen-bonded waters. Hydronium cations of this composition had never been reported previously – see next, IYEPEH. For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.
IYEPEH (Fig. 3, Ref. [7]) has the interesting feature of also having the previously unknown composition H11O5+, but, more interesting is the fact that it is a cyclic species of composition tris(tetraphenylphosphonium) 25,26,27,28-tetrahydroxy-calix(4)arene-5,11,17,23-tetrasulfonate clathrate oxonium undecahydrate, [C28H20O16S4]4[3(C24H20P+)·(H3O+)11(H2O)]. Again, note that the hydronium cation is formulated as being H3O+.
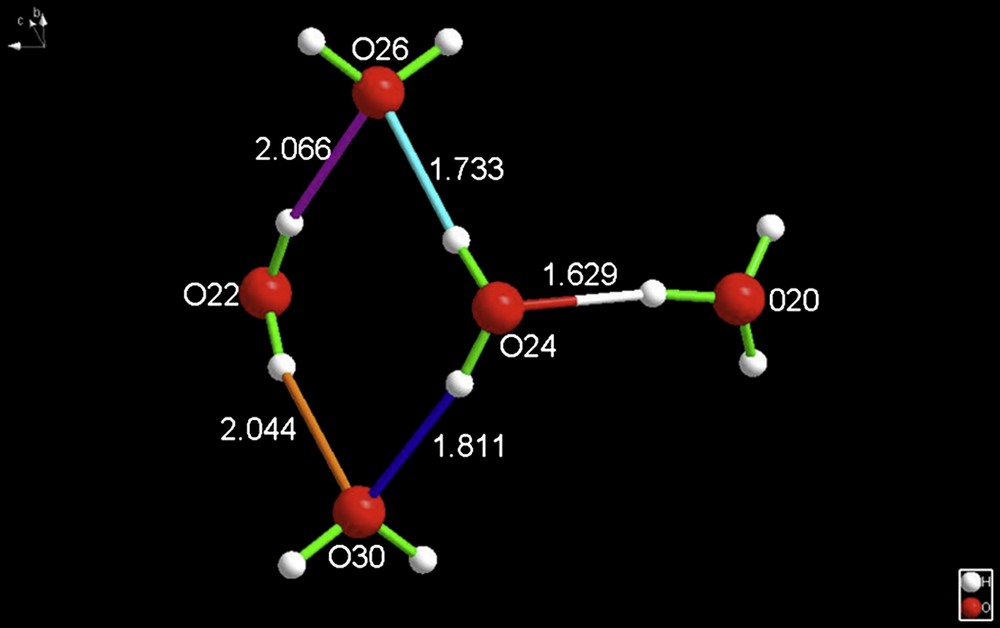
A decidedly unique feature of the hydronium cation is the fact that there is an exocyclic H3O+ cation asymmetrically hydrogen bonded to a ring of four waters. Such an arrangement had not been reported previously. O20 is the hydronium cation oxygen. For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.
The final species discovered is NEBDII [8] which was described as being inverted cucurbit(6)uril oxonium chloride hydrate, [C36H36N24O12·8.7(H2O), H3O+]Cl. Once more, note the formulation of the hydronium cation. The species is, in reality, shown in Fig. 4, and has composition H15O7+ – a cation of such composition had never been previously reported.
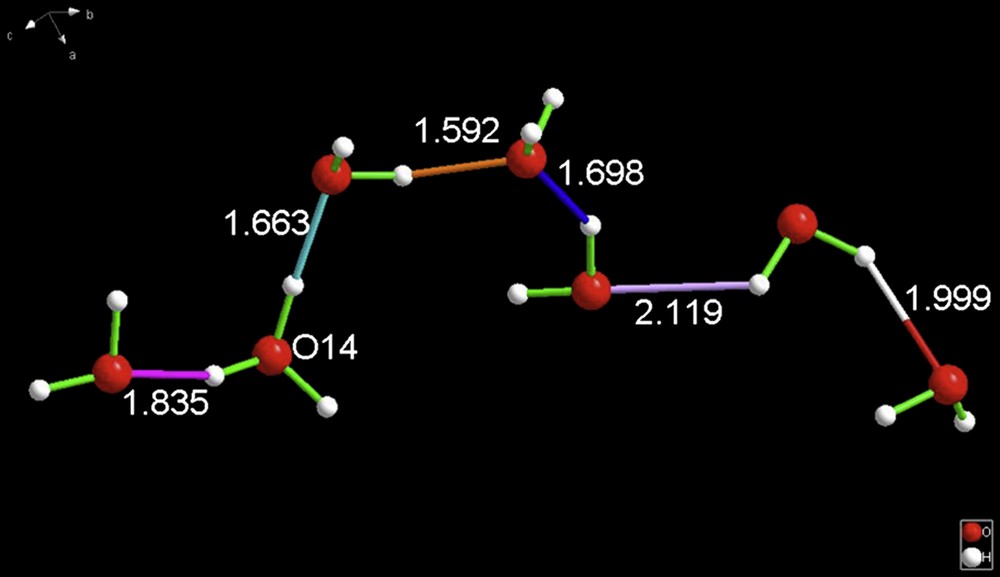
In analogy with BEXFEQ (see above), NEBDII is a highly asymmetric, acyclic cation. O14 is the H3O+ fragment. As in BEXFEQ, it has hydrogen-bonded water at one end (H3O+ is the proton donor on both sides); the other end is a long chain of five hydrogen-bonded waters. For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.
4 Conclusions
- 1. As can be appreciated from the figures displayed above, the hydrogen bonds holding these assemblies are quite substantial – a limit of 2.20 Å was imposed on the bonds accepted in drawing the figures. Therefore, it is expected that these hydronium cations are very stable, even as isolated species, free of their crystalline lattice supports.
- 2. Nature seems to have an inexhaustible ability to manipulate a single type of bond (hydrogen bond) into a never ending assembly of hydronium cations, often even into geometrical isomers. The new ones reported here have never been described before, specially IYEPEH (Fig. 3) that is not only cyclic but has an exocyclic hydronium cation attached to it.
1 Cambridge Structural Database, Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ UK, Tel.: +44 1223 336408. Available from http://www.ccdc.cam.ac.uk released in April of 2006.
2 A number of six capital letters are used to designate species discussed in the article. Those words are the REFCODES used by the Cambridge Structural Base to identify the species they catalog.


