1 Introduction
Linear π-conjugated oligomers and polymers have been extensively investigated for targeting molecular electronics [1,2]. Among these, oligo- and polythiophenes seem to be one of the best candidates for using in components of electronic and optoelectronic devices [3,4], because oxidation of oligo- and polythiophenes produces highly electroconductive composites caused by polaron, bipolaron, and π-dimer formations [5]. Furthermore, oligothiophenes can be employed for an electroactive conjugated molecular wire with high rigidity and thermal stability [6–8]. The rigidity of oligothiophenes is also of great advantage for their use as a conjugated spacer in donor–acceptor diads and triads [9].
In contrast to linear oligomers, fully conjugated macrocyclic oligoarylenes, heteroarylenes, and their π-expanded counterparts are regarded as an infinite π-conjugated system with an inner cavity [10]. Therefore, these macrocycles have attracted considerable attention due to their effective conjugation length and unusual electronic properties [11]. Recently, Bäuerle and coworkers have synthesized a series of cyclic oligothiophenes and characterized their fantastic molecular structures using X-ray and STM analyses [12]. We are particularly interested in shape-persistent or semishape-persistent macrocycles with extremely large molecular diameters. Although fully conjugated macrocycles within 30 Å molecular size have been reported by several groups [13–16], few π-expanded cyclic oligophenylenes and oligoheteroarylenes with full conjugation have been studied to date [17]. Recently, Mayor and Didschies have reported the largest conjugated molecular ring with a diameter of 11.8 nm [17]. This molecule has a fully conjugated periphery composed of ethynylene, butadienylidene, 1,4-thienylene, and 1,4-phenylene units, only exhibiting weak cyclic conjugation.
Here, the synthesis, novel molecular structures, electronic and optoelectronic properties, and supramolecular chemistry of π-expanded cyclic oligothiophenes are summarized to provide an introduction to the new field of giant macrocyclic oligothiophenes [18].
2 Giant macrocyclic oligothiophenes
2.1 Molecular design and synthesis
π-Expanded oligothiophenes 1–3 are shape-persistent or semishape-persistent because of their planar thiophene, acetylene, and ethylene units (Fig. 1). We planned the synthesis of 2 and 3 using successive Sonogashira and McMurry coupling reactions. Thus, 2a–2e and 3a–3d are suitable target molecules for constructing giant macrocycles with full π-conjugation, because 2a–2e and 3a–3d are expected to have a nearly circular shape with 30–80 Å molecular diameters and 18–60 Å inner cavities.1 In addition, the calculated structures of 2a–2e and 3a–3d have no severe ring strain.
Although no host–guest complex derived from giant macrocycles of oligothiophenes has been reported to date, sulfur atoms having a dipole may incorporate an ion or small molecule in the macrocyclic rings like thiacrown ethers [19]. Furthermore, π-expanded oligothiophenes can be envisaged as self-assembling into nanostructures such as nanowires, nanotubes, and nanoparticles using S…S and π–π interactions [20]. Giant macrocycles with long alkyl chains usually form an amorphous solid or liquid crystals different from well-known macrocyclic oligophenylenes which lead to the formation of crystal structures with π–π stacking interaction. Thus, reducing the dimensionality of macrocycles having non-H-bonding interactions from the three-dimensional crystal structure into the one-dimensional columnar structure leads to the formation of interesting self-assembling nanostructures. Our π-expanded oligothiophene macrocycles differ from macrocyclic oligoheteroarenes, such as macrocyclic oligothiophenes and oligopyrroles [12,21], in that our oligothiophene macrocycles can flexibly form self-assembling nanostructures.
Syntheses of 2a–2e and 3a–3d are summarized in Schemes 1 and 2. Intermolecular cyclization of 4 under modified McMurry conditions produces a mixture of cyclic oligomers, which can be separated easily by gel permeation chromatography on polystyrene with toluene or chloroform as an eluent to afford the 30π-dimer 2a, 90π-trimer 2b, 120π-tetramer 2c, 150π-pentamer 2d, and 180π-hexamer 2e in 32, 9.4, 6.2, 3.9, and 2.3% yields, respectively, together with a 1:3 mixture of the E,Z- and Z,Z-isomers of 2a (7%) (Scheme 1). Since 2a crystallizes from chloroform–heptane, pure 2a can be isolated from the mixture of stereoisomers. For the synthesis of cyclo[10](2,5-thienylene–ethynylene) 1a, bromination–dehydrobromination of 2a produces 1a in 36% yield. All macrocycles 1a and 2a–2e are stable in the crystalline state and can be stored in air at room temperature for a year. VT NMR spectroscopic studies revealed that 1a and 2a–2e show symmetrical structures even at −60 °C, reflecting a rapid conformational change in solutions.

Synthesis of 2a–2e.

Synthesis of 3a–3d.
Intermolecular McMurry cyclization of 5 produces the 72π-dimer 3a, 108π-trimer 3b, 144π-tetramer 3c, and 180π-pentamer 3d in 39, 8.3, 2.5, and 1.2% yields, respectively (Scheme 2). In addition, 216π-hexamer 3e was isolated in 0.2% yield. Unfortunately, however, conversion of 3a to 1b with bromination–dehydrobromination procedure afforded the desired 1b in a trace yield. Therefore, 3e and 1b were not fully characterized. All macrocycles are stable in air at room temperature.
2.2 X-ray structural analyses
Fantastic molecular structures of 2a and 3a have been determined by X-ray analysis (Figs. 2 and 3). A single crystal of 2a from chloroform–heptane contains 1.5 molar ratio of heptane to 2a. As shown in Fig. 2a, the 10 thiophene rings connected by unsaturated carbon–carbon linkages are arranged circularly with all the sulfur atoms in s-cisoid thiophene rings directed towards the inside. This s-cisoid conformation makes the backbone curl and forms a full circle. The intramolecular distances between two sulfur atoms of thiophene rings are 19.9 and 17.1 Å. The large cavity of 2a is filled by central heptane and edging butyl groups of neighboring molecules, and the mutual sharing of the butyl groups of the neighboring molecules causes its frame to have a slightly bent chair-like conformation (Fig. 2b). Heptane molecules incorporated in the ring are considerably mobile, despite low-temperature X-ray analysis at −180 °C. The X-ray analysis reveals that 2a serves as a host molecule to alkane guests. Linear alkanes like hexane and octane also incorporate in 2a to form single crystals.

X-ray crystal structure of 2a. (a) Top view. (b) Packing diagram with the included heptane. Butyl groups are omitted for clarity.
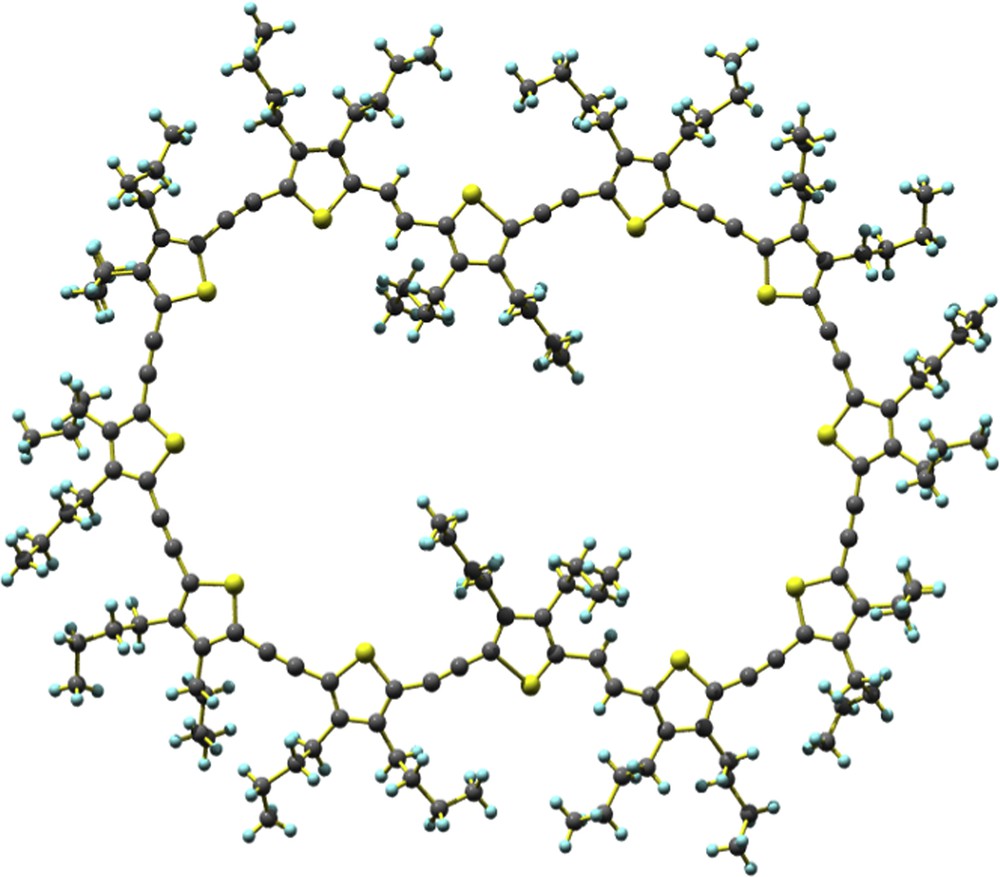
X-ray crystal structure of 3a (top view).
A single crystal suitable for X-ray analysis was obtained from chloroform–decane (Fig. 3). Although 2a composed of 10 thiophene units has a nearly round shape structure with all thiophene units in cisoid form, 3a composed of 12 thiophene units is narrow in the middle and has two thiophene units in a transoid form so as to fill the center of the cycle. The resultant small cavities are filled with neighboring butyl groups, and 3a has a slightly bent chair-like structure. As a result, the single crystal involves no solvent molecule.
3 Giant macrocycles as functional materials
3.1 Redox behavior
All giant macrocycles 1a, 2a–2e, and 3a–3d behave as π-donors with fairly low oxidation potentials, although linear oligo(thienylene–ethynylene)s exhibit lower π-donor ability than common linear oligothiophenes [22]. Cyclic voltammetric (CV) analyses of 1a and 2a show them to exhibit four and three reversible waves, respectively, whereas 2b–2e and 3a–3d show two reversible waves. Therefore, smaller macrocycles exhibit stronger electronic interaction corresponding to on-site Coulombic repulsion, but macrocycles larger than 72π system show only a small on-site Coulombic repulsion in the polycationic states.
Oligo- and polythiophenes are easily oxidized with electron acceptors to form corresponding radical salts. One important concept to realize high electric conductivity is π–π stacking and π-dimer formation of cationic species derived from oligo- and polythiophenes. Although linear poly(thienylene–ethynylene)s seem to produce no electroconductive cationic species owing to difficulty in the formation of stable cationic species, cyclic 2a–2e and 3a–3d produce stable cationic species by oxidation and hence can form an electroconductive oxidation state. Actually, doping of 2a, 2b, and 2c with iodine vapor resulted in the formation of black materials with moderate conductivities (2a: σrt = 1.86 × 10−3 S cm−1; 2b: σrt = 2.63 × 10−3 S cm−1; 2c: σrt = 2.03 × 10−3 S cm−1, measured by 2 probe method). All electric conductivities of the black materials increased after exposing 2a, 2b, and 2c to iodine vapor for 5–70 min, though their conductivities gradually decreased to less than one-half of their maximum values after additional exposure.
3.2 Optical properties
Absorption and emission spectra of giant macrocycles 2a–2e and 3a–3d measured in CH2Cl2 exhibit a unique feature (Table 1). As has been reported previously, a series of linear oligo(thienylene–ethynylene)s up to 16-mer exhibited near saturation for the absorption maximum at the octamer stage [6,23]. Therefore, it has been concluded that doubling the conjugation length from octamer to 16-mer causes little change in the absorption maximum. In contrast, the expanded cyclic oligothiophenes 1a, 2a–2e, and 3a–3b except for 3c and 3d exhibit a redshift of the longest absorption maxima with increasing ring size, reflecting an almost full conjugation through the rings (Fig. 4). In the case of emission spectra, linear oligo(thienylene–ethynylene)s were reported to exhibit two major emission bands based on the vibronic structure of 0-0 and 0-1 transitions [24]. As shown in Table 1, fluorescence spectra of 1–5 show two major emissions at almost the same wavelengths (559–562 and 600–606 nm) with a large Stokes shift of 72–157 nm. The separation of two emissions in the fluorescence spectra corresponds to the vibrational energy gap.
Absorption coefficients and fluorescence quantum yields of 1a, 2a–2e, and 3a–3d in CH2Cl2.
| Compd. | Absorption | Fluorescence | ||
| λmax [nm] | ɛ [M−1 cm−1] | λmax [nm] | ΦFa | |
| 1a (60π) | 442 | 235,000 | 537, 579 | 0.062 |
| 2a (60π) | 447 | 249,000 | 560, 604 | 0.069 |
| 3a (72π) | 452 | 295,000 | 562, 606 | 0.11 |
| 2b (90π) | 469 | 311,000 | 562, 605 | 0.084 |
| 3b (108π) | 473 | 418,000 | 560, 603 | 0.085 |
| 2c (120π) | 479 | 405,000 | 560, 602 | 0.11 |
| 3c (144π) | 478 | 528,000 | 559, 600 | 0.10 |
| 2d (150π) | 485 | 551,000 | 560, 603 | 0.089 |
| 3d (180π) | 480 | 618,000 | 559, 600 | 0.095 |
| 2e (180π) | 488 | 639,000 | 560, 600 | 0.086 |
a Fluorescence quantum yields (ΦF) were determined by comparison with quinine sulfate in 0.5 M H2SO4 (ΦF = 0.51).
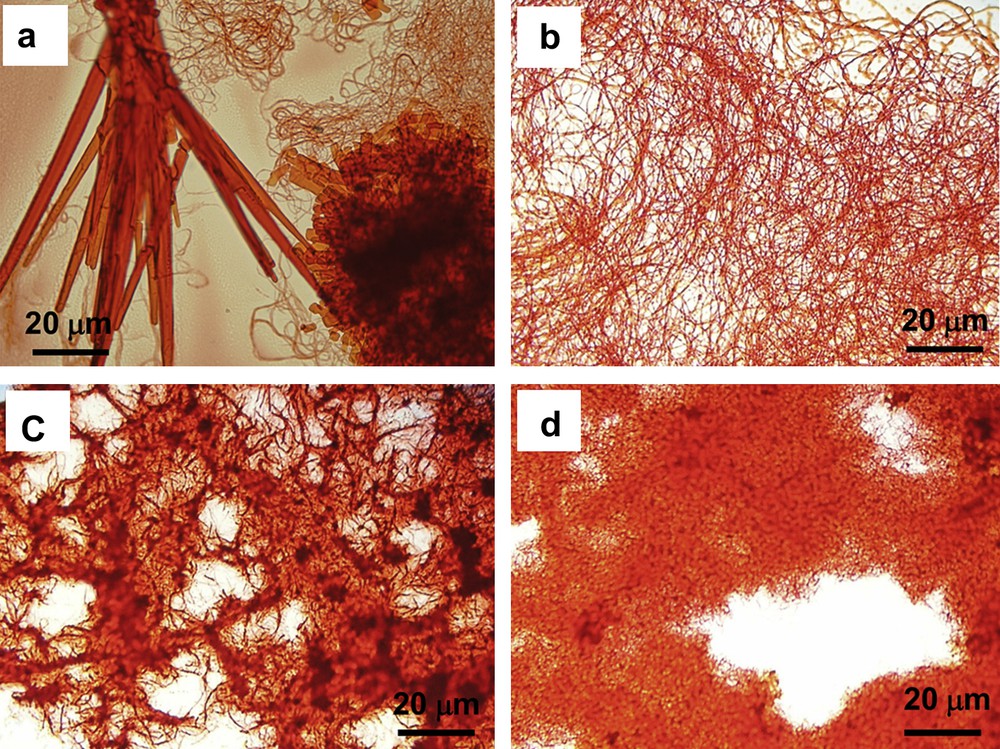
Microscopic images of 2b (a), 2c (b), 2d (c) and 2e (d) with 1000× magnification.
Among giant macrocycles, the two-photon properties of 3b–3d were investigated [18c]. The two-photon absorption cross-sections (δmax) are 3a: 15,100 GM, 3b: 66,700 GM, 3c: 82,600 GM, and 3d: 107,800 GM. Therefore, increasing ring size and π-character from 3a (72π) to 3b (108π), 3a to 3c (144π), and 3a to 3d (180π) result in 4.4-, 5.5-, and 7.1-fold amplifications of the maximum two-photon absorption cross-section, respectively. These large enhancements of two-photon absorption cross-section are due to intramolecular interactions among these giant macrocycles. Note that the increasing π-conjugation leads to an increase in the two-photon absorption cross-section with magnitudes as high as 100,000 GM in the visible spectral region.
3.3 Nanostructures and fiber formation
Interestingly, giant macrocycles 2b–2e and 3b–3d form complex supramolecular structures owing to their weak amphiphilic properties. Although 2a and 3a form single crystals by recrystallization, 2b forms microcrystals and nanowires from ethyl acetate/octane, and 2c forms nanowires from ethyl acetate/chloroform (Fig. 4a and b). In contrast, 2d and 2e form nanoparticles from ethyl acetate/chloroform (Fig. 4c and d). All microcrystals, nanowires, and nanoparticles of 2b–2e contain no solvents, different from single crystals of 2a. The aggregates of 2b and 2c have well-defined fibrous structures with 100–200 nm thickness, whereas 2d and 2e form chained lumps of 300–800 nm size. Similarly, 3b has a petal structure, whereas 3c and 3d form chained lumps. Because 2b–2e and 3b–3d have solvophilic and solvophobic moieties, the amphiphilic properties result in microscopic separation which leads to the formation of nanofibers and nanoparticles.
4 Conclusion
McMurry coupling reaction was found to be a versatile tool for the construction of giant macrocycles 2a (60π)–2e (180π) and 3a (72π)–3d (180π). Giant macrocycles 1a, 2a–2e, and 3a–3b exhibit a redshift of the longest absorption maxima with increasing ring size owing to cyclic conjugation through the rings. In contrast, emissions in the fluorescence spectra of 1a, 2a–2e, and 3a–3d are observed at almost the same position, reflecting the similar HOMO–LUMO gaps. The absorption and emission spectra of 6 exhibit a blue shift as compared with 1. The infinite cyclic 1a, 2a–2e, and 3a–3d can be easily oxidized to show reversible redox potentials by CV analysis, and doping of 2a–2c with iodine vapor forms semiconductors. X-ray analysis has indicated that 2a adopts an almost planar round shape with s-cisoid thiophene rings, and that a 1.5 molar ratio of heptane to 2a locates inside in the cavity. In contrast, 3a is narrow in the middle and has two thiophene units in s-transoid form filling the center of the cycle. Therefore, the single crystal of 3a involves no solvent molecule. One of the most interesting properties of giant macrocycles is their different morphology, when these compounds are precipitated from aprotic solvents. Thus, 2a and 3a form single crystals from chloroform–heptane/decane, whereas 2b and 2c yield nanowires, and 2d–2e and 5 form microscale lumps. Another remarkable property of giant macrocycles is the inherent large two-photon absorption cross-section. The extremely large nonlinear optical effects of giant macrocycles will open up a new window on two-photon properties.
Acknowledgements
This work was partly supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan and by CTREST of JST (Japan Science and Technology Corporation). I would like to thank all researchers, including collaborators, colleagues and coworkers, whose work has been presented in this chapter. I would also like to thank Dr. Masaharu Akiba (Fujifilm Corporation) for the preliminary measurements of the two-photon properties of giant macrocycles.



