1 Introduction
Native plants have been reported to have antimicrobial and antioxidant properties for centuries, and indigenous plants have been used in herbal medicine to cure various diseases [1]. Many kinds of diseases have been treated with herbal remedies since ancient times. Herbal remedies are still being used extensively in many countries. Therefore, research on biologically active extracts and compounds from natural sources has been of great interest to scientists in an attempt to discover new sources for drugs that may be useful in combating infectious diseases. Many authors reported that it was necessary to establish a rational relationship between chemical, biological and therapeutic activities in traditional medicine [2,3]. In recent years, there has been a resurgence of interest in evaluating plants possessing antibacterial activities for various diseases [4,5]. A number of studies dealing with antimicrobial screening of extracts medicinal plants have been conducted [6,7]. Current research on free radicals confirms the essential role played by rich foods in antioxidants in the prevention of cardiovascular diseases and cancers [8,9], neurodegenerative diseases, including Parkinson's and Alzheimer's diseases [10] and inflammation problems of aging cutaneous cells [11,12].
Tunisian natural herbs are being more widely used on a commercial scale in the food industry, in traditional medicine, and for their flavoring properties [13]. Wild plants, including Henophyton deserti, are widely used as medicines by populations that inhabit the rural parts of southern Tunisia. H. deserti Coss. & Durieu, Synonym Oudneya Africana R. Brown, is an endemic Saharan plant of the Brassicaceae alt. Cruciferae family. This shrub has great potentialities to provide different products and services as forage, traditional medicine, halting desert encroachment and stabilizing sand dunes [14]. The seeds and leaves of these plants are usually prepared and used to treat digestive problems, arthritis, colds and flu, fevers, irritability, and scorpion bites [13,15]. Until recently, this species has only been studied for its chemical properties by Stocker et al. [16]. This study showed a strong inhibitory effect of phenolic extracts of H. deserti on the porcine kidney acylase-I activity.
Apart from this study, we could not find any other inquiry into biological activities of this genus. Therefore, the aim of this study is to evaluate in vitro antimicrobial and antioxidant activities of H. deserti in addition to their chemical compositions of the main fatty acids and phenolic compounds such as flavanoids by liquid chromatography/mass spectrometry (LC/MS) and gas chromatography/mass spectrometry (GC/MS).
2 Materials and methods
2.1 Plant material
Aerial parts and mature seeds of H. deserti were collected in March and June 2005, respectively, from Bir Soltan located in the Grand Erg Oriental (33° 17, 05 N; 09° 42, 41 E). This species was identified by Pr. Mohamed Chaieb. Voucher specimens were deposited at the Herbarium of the laboratory of Biology & Ecophysiology of Plants, Department of Science, University of Sfax, Tunisia.
2.2 Extraction procedures
The dried powdered seeds (14.5 g) and leaves (52.54 g) of H. deserti were subjected to successive solvent extraction (hexane, ethyl acetate and methanol) for 12 h under a continuous reflux setup in a Soxhlet extractor. The extracts were concentrated under reduced pressure (rota vapour) then preserved at +4 °C. Residues of plant extracts were dissolved in 50 mg ml−1 ethanol/water (20/80, v/v) before use in antimicrobial assay.
2.3 GC/MS analysis
An aliquot of 1 ml extract was injected, splitless, into the GC/MS (5975B inert MSD Agilent). The data were displayed on a DB-5MS column, 30 m in length, 0.25 mm i.d. and 0.25 mm in thickness (Agilent Technologies, J&W Scientific Products, USA). The carrier gas was helium. GC oven temperature started at 100 °C and was held for 1 min at 260 °C and then for 10 min with program rate 4 °C min−1. The injector and detector temperatures were set at 250 and 230 °C, respectively. The mass range was scanned from 50 to 550 amu. The control of the GC/MS system and the data peak processing were carried out by means of the MSDCHEM software.
2.4 Fatty acid determination (GC)
The fatty acid composition of the oils was determined by gas chromatography (GC) as fatty acid methyl esters (FAMEs). FAMEs were prepared by saponification/methylation with sodium methylate according to the European Union Commission modified Regulation EEC 2568/91. A chromatographic analysis was performed in a SHIMADZU set 17A Series II gas chromatograph using a capillary column (stabilwax, Restek). The column temperature was isothermal at 180 °C and the injector 230 °C and detector temperatures were 250 °C. Fatty acids were identified by comparing retention times with standard compounds and expressed as percentage of fatty acid methyl esters.
2.5 LC–MS/MS analysis
The LC–MS/MS experiments were carried out with an Agilent 1100 LC system consisting of degasser, binary pump, auto sampler, and column heater. The column outlet was coupled to an Agilent MSD Ion Trap XCT mass spectrometer equipped with an ESI ion source. Data acquisition and mass spectrometric evaluation were carried out in a personal computer with Data Analysis software (Chemstations). For the chromatographic separation, a Zorbax 300 Å Extend-C-18 Column (2.1 × 150 mm) was used. The column was held at 95% solvent A (0.1% formic acid in water) and 5% solvent B (0.1% formic acid in ACN) for 1 min, followed by an 11 min step gradient from 5% B to 100% B, then it was kept for 4 min with 100% B, finally, the elution was achieved with a linear gradient from 100% B to 5% B for 2 min.
The flow rate was 200 μL min−1 and the injection volume 5 μL. The following parameters were used throughout all MS experiments: for electrospray ionisation with positive ion polarity the capillary voltage was set to 3.5 kV, the drying temperature to 350 °C, the nebulizer pressure to 40 psi, and the drying gas flow to 10 L min−1. The maximum accumulation time was 50 ms, the scan speed was 26,000 m z−1 s−1 (ultra scan mode) and the fragmentation time was 30 ms.
The flavonoids and other phenolics were identified using a combination of high performance liquid chromatography (HPLC) with diode array detection and liquid chromatography with atmospheric pressure chemical ionization mass spectrometry (ESI-LC–MS/MS) on the basis of their ultraviolet (UV) spectra, mass spectra and by comparison of the spectra with those of available authentic standards.
2.6 Determination of total phenolics
Total phenol content of extracts was determined using the Folin-Ciocalteu technique [17]. Briefly, a 50 μl aliquot of different extracts was assayed with 250 μl of Folin reagent and 500 μl sodium carbonate (20%, w/v). The mixture was vortexed and diluted with water to a final volume of 5 ml. After incubation for 30 min at room temperature, the absorbance was read at 765 nm and total phenols in the extracts were expressed as gallic acid equivalents (GAE), using a calibration curve of a freshly prepared gallic acid solution. We performed 10 determinations (n = 10). For the gallic acid, the curve absorbance versus concentration is described by the equation y = 0.0012 x − 0.0345 (R2 = 0.9997).
2.7 Determination of total flavonoids
Total flavonoids were measured by the colorimetric assay developed by Zhishen et al. [18]. One ml aliquot of appropriately diluted sample or standard solution of quercetin (20, 40, 60, 80 and 100 mg l−1) was added to a 10 ml volumetric flask containing 4 ml ddH2O. At zero time, 0.3 ml 5% NaNO2 was added to the flask. After 5 min, 0.3 ml 10% AlCl3 was added. At 6 min, 2 ml 1 M NaOH was added to the mixture. Immediately, the reaction flask was diluted to volume with the addition of 2.4 ml of ddH2O and thoroughly mixed. Absorbance of the mixture – pink colour – was determined at 510 nm compared to control water. Total flavonoid extracts were expressed as fresh weight mg/100 g quercetin equivalents (QE). Samples were analysed in five replications.
2.8 Determination of antioxidant activity using free radical scavenging activity (DPPH)
Different concentrations (5, 10, 20, and 40 μl equivalent to 5, 10, 20, and 40 ppm) of extracts and 2,6-di-tert-butyl-4-méthylphénol (BHT) were taken in different test tubes. The volume of the sample/BHT was adjusted to 100 μl by adding MeOH. Methanolic solution (5.0 ml) of 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) (100 μM) was added to these tubes and shaken vigorously. The tubes were allowed to stand at 27 °C for 20 min [19]. The control was prepared as above without extracts and MeOH was used for the baseline correction. The changes in the absorbance of the extracts and BHT were measured at 517 nm. Radical scavenging activity was expressed as the inhibition percentage and was calculated using the following formula:
2.9 Antioxidant assay by β-carotene-linoleate model system
The procedure of Hidalgo [20] was followed for testing antioxidant activity (AA) of the extracts with a minor modification. The assay reagent was prepared as follows. β-Carotene (0.2 mg) in 0.5 ml of chloroform was added to 20 mg of linoleic acid and 200 mg of Tween 40 were mixed. The Tween 40 was warmed in a 50 °C water bath before use. The chloroform was removed at 40 °C under vacuum using a rotary evaporator. The resulting solution was immediately diluted with 10 ml of triple-distilled water and the emulsion was well mixed for 1 min. The emulsion was further diluted with 40 ml of oxygenated water before being used. A 4 ml aliquot of this reagent was transferred into different tubes containing 0.2 ml of the desired amount of antioxidant in ethanol.
A control sample consisting of 0.2 ml of ethanol and 4 ml of emulsion was prepared. Readings of all samples were taken immediately (t = 0) and at 15 min intervals for 2 h (t = 120). The tubes were placed in a 50 °C water bath between measurements. All determinations were performed in duplicate. Colour measurement was recorded until the β-carotene colour disappeared. The antioxidant activity (AA) of the isolated compound was evaluated in term of bleaching the β-carotene using the formula of Hidalgo [20].
2.10 Antimicrobial assay
Antimicrobial assay was achieved on one filamentous fungus Aspergillus niger CIP 1431.83, one yeast Candida albicans CIP 48.72 and five bacteria belonging to the two groups of the Gram staining, the empirical method of differentiating bacterial species into two large groups (Gram-positive and Gram-negative) based on the chemical and physical properties of their cell walls. Two Gram+ bacteria Staphylococus aureus subsp. aureus CIP 4.83 and Bacillus subtilis CIP 52.62 and three Gram− bacteria Pseudomonas aeruginosa CIP 82.118, Escherichia coli CIP 53.126, Salmonella enterica CIP 80.39. Microorganisms were obtained from the culture collection of the Pasteur Institute, France. The bacteria were cultivated in Nutrient Broth (NB) or Nutrient Agar (NA) (Difco) at 37 ± 0.2 °C for Staphylococus, Escherichia and Salmonella, and at 30 ± 0.2 °C for Pseudomonas, Bacillus. Fungi and yeasts were cultured on Malt Extract Broth (MEB) or Agar (MEA) (Difco) at 28 ± 0.2 °C. The cultures of bacteria and fungi were maintained in their appropriate agar slants at 4 °C throughout the study and used as stock cultures.
Inocula were prepared by adjusting the turbidity of each bacterial and yeast cultures or fungal spore suspension, to reach an optical comparison to that of a 0.5 McFarland standard, resulting in a suspension containing approximately 1 to 5 × 108 CFU ml−1.
The extracts were tested with the disc-diffusion method [21]. The bacterial and yeast cultures in exponential phase of growth or fungal spore solution (100 μl), were spread on sterile NA or MEA plates, after which 6 mm-diameter discs (sterile blank), impregnated with the extract to be tested (40 μl of the dried extract redissolved in ethanol/water (20/80, v/v) at 50 mg ml−1), were placed on the plates. The in vitro antimicrobial activities of these extracts tested at the concentration of (2 mg per disc) against the standard microorganisms. Their activities were qualitatively assessed by the presence or absence of inhibition zones and zone diameters. Respective solvent ethanol/water (20/80, v/v) without plant extract served as negative control. PolymyxineB antibiotic biodisc (bioMerieux sa) was used as reference or positive control for the estimation of H. deserti extracts antimicrobial activities with the disc diffusion method.
The plates were incubated for 24 h or 3 days at 28, 30 or 37 °C according to the optimum growth temperature of the microbial strain, under aerobic conditions and the diameter of the inhibition zone around each disc was then measured and recorded.
2.11 Minimum Inhibitory Concentration (MIC)
The MIC was determined with the broth dilution (National Committee for Clinical Laboratory Standards [NCCLS]) modified method [22]. The reconstituted extract was serially diluted 2-fold in Nutrient Broth (Difco) for bacteria or Malt Extract Broth (MEB) for C. albicans. Duplicate tubes of each dilution (20, 10, 5, 2.5, 1.25, 0.625, 0.313, 0.156, 0.078, 0.039, and 0.02 mg ml−1) were inoculated with about 106 CFU ml−1 of the bacterial and yeast cultures in exponential phase of growth. The tubes were incubated at the appropriate temperature of each strain for 18 hours. A tube containing only broth inoculated with the organism and kept at +4 °C in a refrigerator overnight was used as standard for the determination of complete inhibition. MIC was taken as the highest dilution (least concentration) of extract showing no detectable growth.
2.12 Statistical analysis
Unless otherwise specified, data were expressed as mean ± standard deviation of triplicate experiments. The relationship between total phenolic, flavonoid contents and antioxidant activity in H. deserti extracts was described as Pearson product–moment correlation coefficient (r). Statistical analysis was performed by one-way Analyses of Variance (Anova) followed by the Tukey's HSD test [23]. Differences were considered statistically significant if p < 0.05.
3 Results and discussion
3.1 Chemical composition of H. deserti extracts
To elucidate the structures of phenolic compound, mainly flavonoids in the H. deserti extract, the methanol extract was analysed by HPLC with diode array detection. HPLC analysis of the fractions showed the presence of peaks with flavonoid-type UV spectra (two bands, λmax of band 1 between 320 and 350 nm and λmax of band 2 between 250 and 270 nm) and interfering peaks of other phenolics. As the flavonoids were present at low concentrations, it was not possible to isolate them in sufficient amounts for identification by NMR spectroscopy. Table 1 lists each of the identified compounds in elution order. The structure assignment of flavonoids for which no standards were available, was based on a systematic search for molecular ions using extracted ion mass chromatograms and comparing those with data in the literature [24,25]. For example, the ESI mass spectrum in positive mode of compound 2 exhibited a base peak [M + H]+ at m/z 595 (Table 1), an intermediate ion at m/z 449 and an aglycone ion at m/z 287. The loss of 146 amu from the pseudomolecular ion represents the sugar rhamnose, and the loss of 162 amu from the intermediate ion is due to the loss of glucose. The λmax of the UV spectrum at 345 and 266 nm suggest that flavonoid 2 is a kaempferol 3-O- glycoside, and the results of the MS and UV spectra combined suggests that compound 2 could be kaempferol 3-O-rhamnosylhexoside. The latter was identified by cochromatography with authentic standards as kaempferol 3-O-rutinoside. Flavonoid 3 showed an [M + H]+ ion at m/z 463 with significant fragments at m/z 301 and m/z 300. The UV spectra of compound 3 suggested that it was diosmetin 3-O-glycosides (λmax 252, 267, 344 nm). This assumption was confirmed by analysis of the extract by thin layer chromatography on cellulose [26] in which its spots do not change colour in UV after fuming with ammonia. The pseudomolecular ion [M + H]+ of compound 4 was at m/z 447 (Table 1), and fragment ion at m/z 285. The UV spectra (λmax at 324 and 268 nm) and MS results combined suggest that compound 4 could be an acacetin 7-O-glycoside (Fig. 1). This identification was confirmed after acid hydrolysis [27] of methanol extract followed by HPLC analysis of the reaction products, indicated that acacetin was present in its aglycone form.
List of detected compounds in H. deserti with their HPLC retention times, UV maximum and mass spectral data.
| No. | Compounds | Retention time (min) | UV λmax (nm) in MeOH | [M + H]+ | MS/MS fragments (m/z) |
| 1 | Rutin | 10.10 | 256, 355 | 611 | 465,303 |
| 2 | Kaempferol 3-O rutinoside | 11.3 | 266, 347 | 595 | 449, 287 |
| 3 | Diosmetin 7-O-Glu | 11.47 | 252, 267, 344 | 463 | 463, 301, 300 |
| 4 | Acacetin 7-O-Glu | 12.10 | 268, 324 | 447 | 447, 285 |
| 5 | M1 | 12.96 | 265, 295 | 447 | 285 |
| 6 | M2 | 13.19 | 310 | 531 | 283 |
| 7 | M3 | 13.97 | 310 | 533 | 497 |
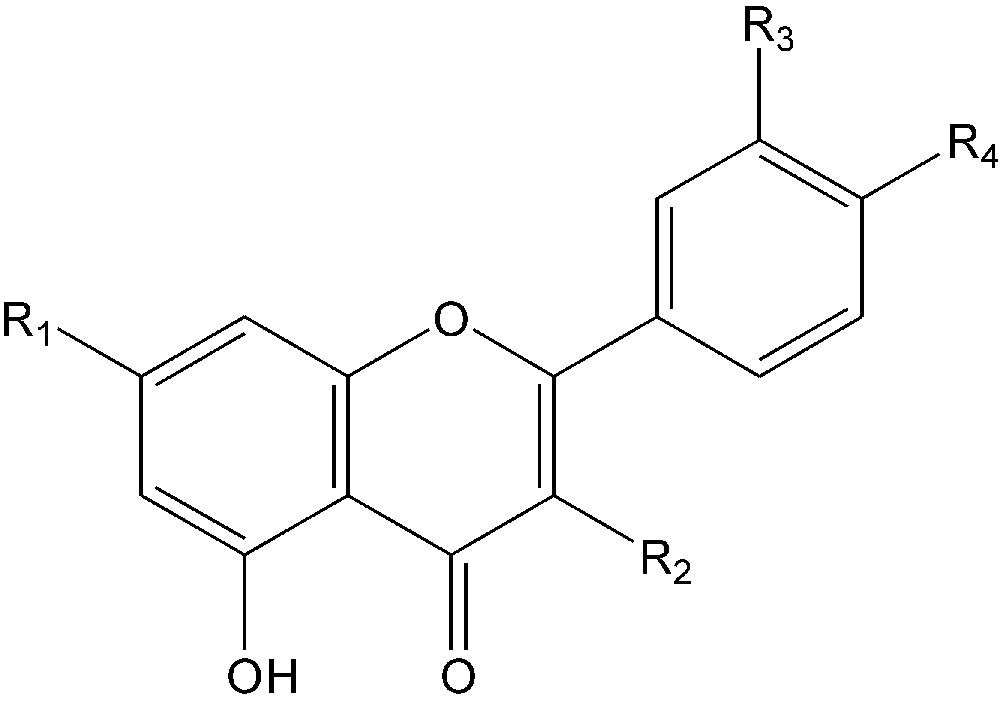
Flavonoids identified in methanol extract of H. deserti.
1: Rutin: R1 = OH; R2 = O-rutinose; R3 = OH; R4 = OH.
2: Kaempferol 3-O-rutinoside: R1 = OH; R2 = O-rutinoside; R3 = H; R4 = OH.
3: Diosmetin 7-O-Glu-: R1 = O-Glu; R2 = H; R3 = OH; R4 = OCH3.
4: Acacetin 7-O-Glu-: R1 = O-Glu; R2 = H; R3 = H; R4 = OCH3.
In the same way, compound 1 was tentatively identified as rutin by comparing its HPLC retention time, UV spectra and mass spectra with the data obtained from standard in-house libraries (Fig. 1). As far as we know, this compound was identified for the first time for this plant. For the non-flavonoid compounds, the mass spectral data indicated that methanol extracts gave a strong [M1 + H]+ ion at m/z 447 and a second prominent [M2 + H]+ ion at m/z 531 with λmax of the UV spectrum at 265 and 295 for M1 and 310 nm for M2. The MS/MS experiments focusing on the fragment generated from the ion m/z = 447 [M1 + H]+ revealed a fragment at m/z 285. The same MS/MS fragmentation pattern was observed for the ion m/z = 531 giving a fragment at m/z 283. This suggests that M1 and M2 present a similar structure. These compounds have been reported previously in the aqueous/ethanol (20/80,v/v) extract of the aerial parts of the same species harvested from the Algerian Sahara [16]. They were identified as 2-(6a,12a-dihydro-6H-5,8,10,12-tetraoxa-benzo[a]cyclopenta[h] fluoren-3-yloxy)-6-hydroxy-methyl-tetrahydro-pyran-3,4,5-triol (M1) and malonic acid mono-[3,4,5-trihydroxy-6-(12a-H-5,8,10,12-tetraoxa-benzo[a] cyclopenta[h]fluoren-3-yloxy)-tetrahydro-pyran-2-ylmethyl] ester (M2). In addition to these two compounds, the mass spectral data shows another peak with prominent mass ions of m/z 533 and 555 corresponding to [M3 + H]+ and [M3 + Na]+, respectively. These data are in agreement with those reported by Stoker et al. [16] who identified the same compound and identified as malonic acid mono-[6-(6a,12a-dihydro-6H-5,8,10,12-tetraoxa-benzo[a] cyclopenta[h]fluoren-3-yloxy)-3,4,5-trihydro- xy-tetrahydro-pyran-2-ylmethyl] ester. Other compounds are present in H. deserti leaf hexane extracts, namely: β-sitosterol (0.97), butyl octadecanoate (0.94), hexadecanoic acid (0.91), and glucose (0.91) with numbers in brackets showing match factors of each compound's mass spectrum to that of the GC/MS library. In addition, fatty acids were identified, in H. deserti seed hexane extracts using GC/MS chromatography (Table 2).
Fatty acids composition of H. deserti seed oil and Soybean oil.
| Fatty acids | H. deserti seed oil (%) | Soybean oil (%)a |
| Palmitic acid C16: 0 | 9.3 ± 0.14 | 9.7 |
| Stearic acid C18: 0 | 2.1 ± 0.03 | 3.9 |
| Oleic acid C18: 1 | 27 ± 0.49 | 25.1 |
| Linoleic acid C18: 2 | 12 ± 0.06 | 54.2 |
| Linolenic acid C18: 3 | 17 ± 0.25 | 5.9 |
| Erucic acid C20: 1 | 8.6 ± 0.05 | – |
| Gadoleic acid C20: 1 | 2.7 ± 0.04 | – |
a Results published by Brodnjak-Voncina et al. [28].
3.2 Oil content and fatty acid composition of H. deserti seeds
The oil of H. deserti seeds harvested from Tunisian Sahara was analysed by GC. The output of the extraction was 11.2%. The high oil content of H. deserti seeds is comparable to several commonly used oil seeds such as rapeseed or soybeans. Therefore, considering the oil content, H. deserti seeds could be interesting for a commercial processing of the oil. Fatty acid composition and content of H. deserti seed oil, expressed as percentage of dry seed weight, are summarised in Table 2. This composition is comparable to the commercial edible Soybean oil [28]. Common fatty acids are present in H. deserti oil, such as saturated palmitic and stearic acids and unsaturated oleic, linoleic and linolenic acids. However, oleic acid is predominant with an amount of 27%. H. deserti seed oil and soybean oils showed a great similarity about the percentage of palmitic, stearic and oleic acids. They differ only by the percentage of the polyunsaturated lionleic and linolenic acids (Table 2). The fatty acids composition of the H. deserti oil did not add new compounds to the known chemotaxonomic classification. Nevertheless, its wealth in polyunsaturated fatty acids such as linoleic and linolenic acids (12 and 17%, respectively), is in favour of a sought-after dietary use. The variation of the fatty acid composition is comparable to the variation given for other commonly used seed oils in the Standard of the Codex Alimentarius [29]. Consequently, this plant could be an interesting source for this fatty acid compared to Macadamia integrifolia and Hippophae rhamnoides seed oils, containing about 20 and more than 40% of it, respectively [30,31]. The saturated fatty acids possessing less important nutritional value contain little palmitic acid (9.3%) and stearic acid (2.1%).
3.3 Total phenolic and flavonoid determination
The yields of total phenolic and total flavonoid extraction of H. deserti are shown in Table 3. The highest total phenolic content was obtained in methanol extract of leaves (275.37 mg GAE g−1 of dry weight extracts), while less phenolic compounds were present in ethyl acetate and hexane seed extracts, with 20.22 ± 1.4 and 19.54 ± 2.6 mg GAE g−1 of dry weight extracts, respectively. This fact is in correlation with polarity of solvents and solubility of phenolic compounds. The total flavonoid content in extracts varied considerably according to the plant organ and solvent used (Table 3). Total flavonoid content using polar and non polar solvents ranged from 181.2 ± 0.6 to 2.03 ± 0.9 mg QE g−1 (dry weight) extract (Table 3). This study showed that total flavonoid content in the methanol extract of leaves exhibited the highest flavonoids content of 181.2 ± 0.6 mg QE g−1 (dry wt.) extract. This was followed by leaf ethyl acetate and seed methanol extracts (45.66 ± 1.3 mg QE g−1 and 38.95 ± 0.3 mg QE 1 g−1 of dry weight extract, respectively). However, total flavonoid content in seed extract using non polar solvent ranged from 7.58 ± 1.02 (dry weight) in ethyl acetate extract to 2.03 ± 0.9 mg QE 1 g−1 (dry weight) in hexane extract (Table 3).
Total phenolic and flavonoid content of H. deserti extracts.
| Extracts | Total phenolic content mg GAE g−1 (dry weight) | Total flavonoid content mg QE g−1 (dry weight) |
| Hexane seed extracts (oil) | 19.54 ± 2.60 | 2.03 ± 0.90 |
| Ethyl acetate seed extracts | 20.22 ± 1.40 | 7.58 ± 1.02 |
| Methanol seed extracts | 134.22 ± 3.80 | 38.95 ± 0.30 |
| Ethyl acetate leaf extract | 152.29 ± 1.20 | 45.66 ± 1.30 |
| Methanol leaf extract | 275.37 ± 2.30 | 181.2 ± 0.60 |
3.4 Antioxidant activity
Each extract was tested for its antioxidant activity, using free radical scavenging DPPH• and β-carotene assays.
Methanol and ethyl acetate leaf extracts possessed the highest DPPH• scavenging activities (85.2 and 67.5%, respectively) followed by methanol seed extract (quenched 61.3% of the radicals in the system) indicating an excellent antioxidant activity on radicals. The hexane and ethyl acetate seed extracts had lower antioxidant activities, and had possessed the lowest DPPH• scavenging activities of 13.5% and 11.7% DPPH radical quenched, respectively.
The antioxidant activities of H. deserti extracts were evaluated also by the β-carotene bleaching method. Table 4 shows the effect of extracts in comparison with BHT. Crude and purified extracts were tested at the 200 ppm level and compared to BHT at the same concentration. This concentration (200 ppm) is the maximum permissible level allowed for synthetic antioxidants concentration of BHT used for food. It can be noticed that methanol seed and leaf extracts showed appreciable antioxidant activities of 62.1 and 88.0% respectively in comparison with the 93.6% inhibition obtained by BHT. However, the ethyl acetate extract showed a powerful antioxidant activity only in leaves (65.5 and 67.5% of inhibition by β-carotene and DPPH systems, respectively).
Antioxidant activity of H. deserti extracts.
| Extracts | Inhibition by β-carotene-linoleate model system (%) | Radical scavenging activity (%) |
| BHT | 93.60 ± 1.29 | 88.30 ± 0.80 |
| Seed hexane extract (oil) | 11.20 ± 1.03 | 13.50 ± 0.03 |
| Seed ethyl acetate extract | 12.60 ± 0.90 | 11.70 ± 1.02 |
| Seed methanol extract | 62.10 ± 0.08 | 61.30 ± 0.50 |
| Leaf ethyl acetate extract | 65.50 ± 1.13 | 67.50 ± 0.80 |
| Leaf methanol extract | 88.00 ± 0.56 | 85.20 ± 0.30 |
Among the H. deserti extracts studied in this work, methanol extracts exhibited the highest antioxidant activities in two investigated systems flowed by ethyl acetate extract. Their powerful activity may be due to their polyphenol content such as flavonoids which react with the DPPH radical by hydrogen atom donation to free radicals, or/and single electron transfer, which is strongly solvent-dependent [32,33].
Generally, polyphenolic compounds were not extracted by hexane [32]. This explained the low antioxidant activities obtained in hexane seed extract. Our results also showed a good correlation (r = 0.971) between total phenolic content and antioxidant activity with a high significance level (P < 0.001) (Table 5). A similar relationship (r = 0.827) with high significance (P < 0.05) was also obtained between total flavonoid content and the antioxidant activity (Table 5). The obtained results showed a linear correlation between total phenolic content in plant extracts and their antioxidant activities as previously described [34,35].
Correlation between the antioxidant activity and phenolic content for aqueous extracts.
| Antioxidant activity | Total phenolic | Total flavonoid | |
| Antioxidant activity | 1 | ||
| Total phenolic | 0,971** | 1 | |
| Total flavonoid | 0,827* | 0,936** | 1 |
* Differences were considered statistically significant if p < 0.05.
** Differences were considered statistically significant if p < 0.001.
3.5 Antimicrobial activity
Table 6 shows the inhibition zone diameter in mm exerted by the five various extracts of seeds and leaves of H. deserti on the seven standard microbial species. H. deserti crude extracts exhibited broad spectral activity against the tested bacteria. All extracts were active on at least one of the bacteria. Ethyl acetate seed extract exhibited combined antibacterial and antifungal activities. All the extracts failed to inhibit the mold A. niger. MIC values of active extracts are shown in Table 7. Among the H. deserti crude extracts tested, hexane seed extract and ethyl acetate seed extract showed the best antibacterial activity with a wider antimicrobial spectrum. Among the extracts tested, only the ethyl acetate seed extract displayed the best activity against C. albicans with an MIC value of 2.5 mg ml−1. Good antibacterial activity against S. aureus was detected with ethyl acetate leaf extract with the lowest MIC value of 0.156 mg ml−1 only. Good antibacterial activity was also detected with hexane seed extract on this Gram + bacterium. B. subtilis was sensitive to the four extracts: methanol leaf extract, hexane seed extract, methanol seed extract and ethyl acetate seed extract, but the MIC values were relatively high (Table 7).
Antimicrobial activity of various extracts of seeds and leaves of H. deserti on standard bacterial and fungal strains by disc diffusion method.
| Diameter of clear zone (mm) | |||||||
| Microbial species | Control − | Control + | E1 | E2 | E3 | E4 | E5 |
| Staphylococus aureus | 0 | 12 | 10 | 0 | 10 | 0 | 10 |
| Pseudomonas aeruginosa | 0 | 10 | 0 | 0 | 10 | 0 | 11 |
| Escherichia coli | 0 | 7 | 0 | 0 | 0 | 0 | 11 |
| Salmonella enterica | 0 | 10 | 0 | 0 | 0 | 0 | 11 |
| Bacillus subtilis | 0 | 11 | 0 | 13 | 11 | 11 | 10 |
| Candida albicans | 0 | 0 | 0 | 0 | 0 | 0 | 11 |
| Aspergillus niger | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Minimum Inhibitory Concentration (MIC) of H. deserti extracts against standard microbial strains.
| MIC (mg ml−1) | |||||
| Microbial species | E1 | E2 | E3 | E4 | E5 |
| Staphylococus aureus | 0.156 | NI | 0.625 | NI | 0.313 |
| Pseudomonas aeruginosa | NI | NI | 1.250 | NI | 5.000 |
| Escherichia coli | NI | NI | NI | NI | 2.500 |
| Salmonella enterica | NI | NI | 1.250 | NI | 1.250 |
| Bacillus subtilis | NI | 2.500 | 1.250 | 2.500 | 2.500 |
| Candida albicans | NI | NI | NI | NI | 2.500 |
| Aspergillus niger | NI | NI | NI | NI | NI |
Poor inhibitory activity was detected against Gram− bacteria with most of the plant extracts tested. As was expected from previous studies [36], few naturally occurring compounds are active against Gram− bacteria, our current findings are in line with these results. Indeed, only hexane seed extract and ethyl acetate seed extract exhibited inhibitory activity against the Gram− bacteria with relatively high MIC values ranging between 1.25 and 5 mg ml−1. Most of the plant extracts tested showed better antibacterial than antifungal activities. This supported the observations made by other investigators that plant pathogenic fungi are more resistant to plant extracts than plant pathogenic bacteria [37]. Heisey and Gorham [38] observed that 13 extracts inhibited the growth of bacteria, while only five extracts inhibited fungal growth.
Antimicrobial extracts from H. deserti can be assumed to be useful in warding off infectious diseases and there is, therefore, a compelling reason to suppose that anti-infective agents could be active against human pathogens as was suggested by folkloric and historical accounts [16]. Infections caused by Candida are among the most difficult to treat with conventional antifungal agents. This plant could thus be used as a drug to improve the treatment of candidose or could be used as a food-conserving additive.
Saharan plants are known for their resistance to stress conditions, and therefore, for their high content in natural antioxidants and their therapeutic virtues [39]. Many studies suggested that the antimicrobial activity of herbs is due to the presence of phenolic compounds containing a polar functional group [40].
4 Conclusions
This study clearly demonstrates that different extracts of H. deserti, an endemic plant of the Saharan zone, showed activity against a range of food-related bacterial and fungal species. Therefore, extracts of this shrub have the potential to extend shelf life or improve food safety. The wide variation in levels and activity range, however, also indicates the application of the taxon will strongly depend on the specific bacterial problem to be addressed. Furthermore, the flavour effects of the extracts on foods and the potential interaction of the antimicrobial compounds with components of food matrices should be investigated.
H. deserti is perfectly adapted to a very arid climate. This shrub is not yet appropriately exploited, except as wild dry pastures or in some folk medicine remedies. It showed a wealth of powerful antioxidant and antimicrobial activities and dietetic fatty acids. Its high phenolic content may be explained by the exposition of the plant to extreme conditions of stress.
This study clearly indicated that it is important to consider both the associated antioxidant activity and phenolic content. Indeed, polar extracts containing higher level of phenolics possessed more powerful antioxidant potential. However, ethyl acetate and hexane seed extracts (non polar extracts) showed the largest antimicrobial spectra.
This plant, mainly its seeds, constitutes an excellent source of fat. Indeed, its elevated unsaturated fatty acids and phenolic compound content makes it a potential source of dietary regime and a protection against numerous diseases and infections.
Acknowledgments
We wish to thank the General Management of Forests (Tunisia) and the World Bank, for the financial support of the study (Don TF 051308 – TUN) and financial support of ministère de l’enseignement supérieur, de la recherche scientifique et de la technologie of Tunisia under contrat programme du laboratoire des bioprocédés. We also wish to thank Miss Sabrina Sérac for her review of English on previous versions of the manuscript.


