The development of methods to attach various elaborated molecules or biomolecules to surfaces has grown a great deal during the last decades due to important progress in nanotechnology [1–3]. In particular, silicon wafers [4,5] have received much attention due to their interesting properties, which allow the use of analysis means (AFM, FTIRATR, ellipsometry), which are not always available with other surfaces such as glass slides. In the course of our work dealing with the functionalisation of silicon wafers by self-assembled monolayer (SAM) of isocyanates and subsequent reactivity of this group [6–8], we were interested in the attachment of trianglamines. Indeed those interesting molecules possess free secondary amino groups suitable for coupling with surface-modified SAM of isocyanates. The coupling of secondary amines of macrocycles with isocyanates is known in solution [9] but it has not yet been tested with supported isocyanates. Trianglamines are new macrocyles which were recently obtained by [3 + 3] cyclocondensation of dialdehydes with trans cyclohexane-1,2-diamine [10] and further reduction of the imines [11]. This methodology allowed creating chiral macrocyles possessing six secondary amines in high yield, without templates, at a preparative scale, and those conditions still represent a challenge in the field of macrocycle synthesis. Following the initial syntheses, wider research with those macrocycles was started [12–21]. Trianglamines were used as receptor for the recognition of aromatic tricarboxylic acid [22], the Zn complexes of trianglamine were shown to catalyze the asymmetric aldol reaction [23], or Henry's reaction [24], and the Cu complex was shown to catalyze Henry's reaction [18]. Nevertheless, many properties of these macrocycles still remain to be explored. In particular, the attachment of these macrocyles to surfaces in order to produce chiral 2D surfaces has not been described so far. Supramolecular interactions between chiral surfaces and chiral molecules have been investigated by chemical force microscopy. McKendry et al. [25,26] have shown that discrimination between chiral molecules attached to the probe tip and to the surface occurred. Following those results, we decided to investigate the adhesion forces between an AFM tip and a SAM both functionalized with trianglamines. In this article, we first studied the attachment of trianglamines to SAM of isocyanates on oxidized silicon wafers. The same reaction was used to attach trianglamines to AFM tips of Si3N4. Preliminary experiments to investigate chiral recognition and forces between enantiomers by chemical force microscopy are presented.
Two types of trianglamines were studied (Scheme 1). These macrocycles were obtained by reacting the corresponding dialdehyde (terephthaldehyde, biphenyl-4,4’-dicarbaldehyde) with enantiomerically pure trans-cyclohexane-1,2-diamine and subsequent reduction of the imines with NaBH4. Both enantiomers of macrocycle 1 and 1′ were synthesized.

Structure of the macrocycles used for the grafting on silicon wafers.
We wanted to develop simple attachment chemistry of these macrocycles in order to prepare SAMs of trianglamines in an easy and efficient way (Scheme 2).
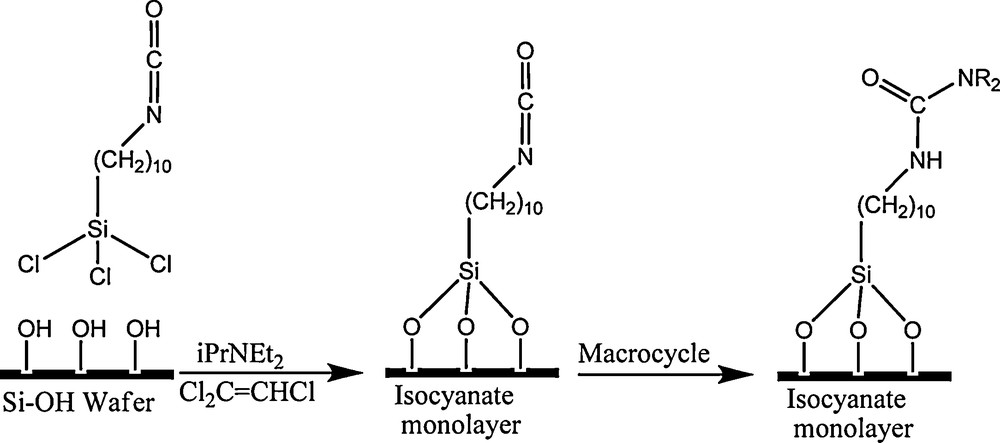
Functionalisation of SAM by trianglamines.
We prepared isocyanate-terminated silicon wafers by reaction of trichlorosilyldecylisocyanate with a silicon wafer crystal (100) with a silica layer of 2.7 nm on the surface. ATR FTIR allowed detecting the isocyanate function and the organization of the decyl chain as a monolayer. The isocyanate monolayer was then reacted with macrocycle 1 in trichloroethylene at RT. Major changes occurred on the ATR FTIR spectrum. The isocyanate band at 2274 cm−1 disappeared within a few seconds and the characteristic bands of the cyclohexyl ring (2925, 2855 cm−1) appeared. The amide I and amide II bands appeared at 1635 and 1535 cm−1, respectively, which is consistent with a total reaction of the supported isocyanate functions with the secondary amines of the macrocycle and formation of urea bonds (Fig. 1).
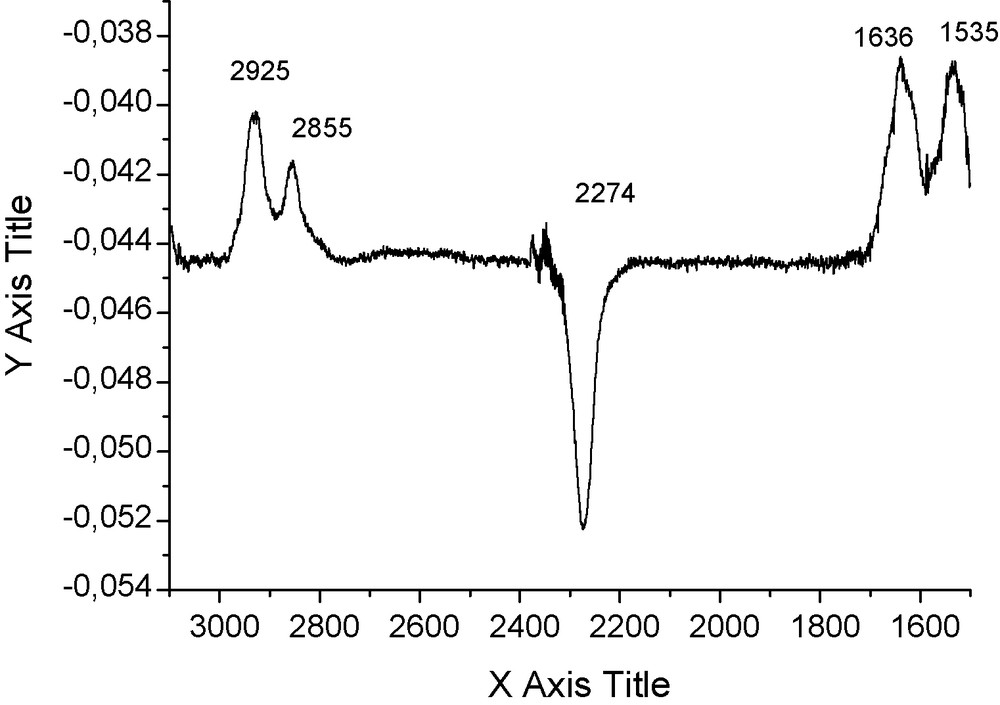
ATRFTIR spectrum of reaction of trianglamine 1 with SAM of isocyanates.
Ellipsometry showed a thickness of the layer of 2.6 nm, in agreement with a monolayer of macrocycle 1 on the surface. Contact angle measurements gave a value of 76° showing a rather hydrophobic surface. Roughness of the wafer was analyzed by AFM, showing a quadratic roughness (Rms) of 1.85 Å, in agreement with a self-assembled monolayer of macrocycle 1 on the wafer, and a homogeneous reaction, no islands were observed. The same results were obtained with macrocycle 1′ (Fig. 2).

AFM of SAM of trianglamine 1.
Reaction of macrocycle 2 with the isocyanate monolayer gave the same ATR FTIR spectrum like macrocycle 1. However, results of ellipsometry measurements with macrocycle 2 showed an increase of the thickness to 3.5 nm. As the aliphatic monolayer has a thickness of 2.1 nm, this means an increase of 14 Å due to the presence of macrocycle 2. Thus, macrocycle 2 did not stand flat on the surface but was rather randomly immobilized. This was further confirmed by AFM experiments, which showed a roughness of 3.1 Å more important than with macrocycle 1. Contact angle measurements increased to 89°, which showed that the aromatic rings of trianglamine 2 were situated on the top of the surface, as the surface became hydrophobic. As a first proof of concept to measure the interactions between chiral surfaces we decided to functionalise the AFM tip made of Si3N4 with a monolayer of trianglamine 1′ (Fig. 3).
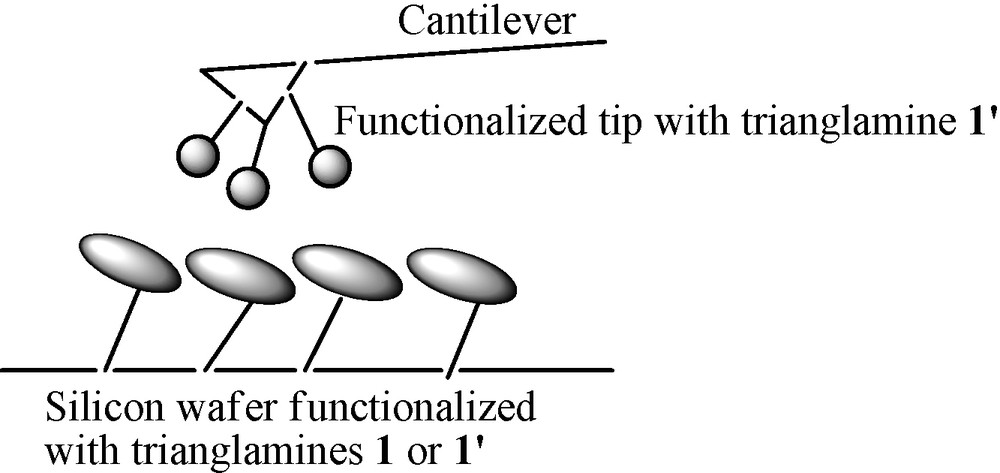
Principle of chiral recognition between the functionalized tip and chiral surface.
The AFM tip was treated with ozone for 30 min to produce OH groups on the surface, then it was grafted with trichlorosilyldecylisocyanate at low concentration, and the isocyanates were further reacted with trianglamine 1′. We first measured adhesion forces between a non-functionalized tip as reference and both surfaces of trianglamines 1 and 1′. Six measurements were made per wafer and the average value with the standard deviation is given. The deflection of the cantilever was recorded at the sample approaches, contacts and withdraws from the cantilever tip, in air. Representative results are shown in Fig. 4. The adhesion forces between a non-functionalized Si3N4 tip and trianglamines 1 and 1′ surfaces gave average values of 11.41 and 11.32 nN with standard deviation of 0.15 and 0.23 respectively. The adhesion forces are similar as there is no chiral differentiation between the tip and the surfaces, and because the same type of SAMs are obtained with enantiomer trianglamines 1 and 1′. The standard deviations were low, which confirmed that the surfaces were homogeneous.

Chemical force microscopy of SAM of trianglamine 1 and 1′, with non-functionalized tip.
When the tip was functionalized with trianglamine 1′, major changes occurred with the adhesion forces as shown in representative Fig. 5. The adhesion forces were stronger than with the non-functionalized tip showing that important interactions occurred between macrocycles and thus that the functionalization of the tip was successful. Surface trianglamine 1 gave an average force of 24.97 nN with standard deviation of 0.46, whereas an average force of 35.86 nN was observed with surface trianglamine 1′ with a standard deviation of 0.45. These differences suggest that chiral discrimination occurred between enantiomers 1 and 1′, and that interaction between enantiomer macrocycles 1 and 1′ were weaker than interactions between trianglamines of the same configuration. The standard deviations were less then 1 nN, which confirmed the preparation of self-assembled monolayers without islands.

Chemical force microscopy of SAM of trianglamine 1 and 1′, with functionalized tip.
In conclusion, we have shown that trianglamines reacted readily with SAM of isocyanates, and that the structure of the resulting SAM depended strongly on the structure of the trianglamine. Chemical force microscopy showed that important interactions occurred between the functionalized tip and the chiral surface, and that interactions were weaker between enantiomer trianglamines than with trianglamines of the same configuration.
1 Experimental section
ATR infrared spectra measured in situ were recorded on a FTIR Perkin-Elmer 2000 spectrometer equipped with narrow band liquid nitrogen cooled MCT detector and a thermoregulated ATR flow cell. The sample compartment was purged with dry air. All the spectra were recorded at a resolution of 1 cm−1, and 128 scans were accumulated. The size of the ATR crystal was 70 × 10 × 1 and 35 reflections were used.
AFM roughness measurements were made on a commercial optical deflection microscope (stand-alone configuration for a large sample, dimension 3100 with a nanoscope IIIa, Veeco) operated in ambient conditions. Measurements were performed in the tapping mode, and commercial silicon nitride cantilever probes with a nominal radius of 5–10 nm and a spring constant in the range of 0.1 N/m were used.
The same instrument was used for adhesion force measurements.
Nucleophiles grafted on Si(100) surfaces:
- • Preparation of oxidized silicon Si(100) surface. First, the native oxide layer was removed by immersion in aqueous HF solution (40%) until total dewetting of the surface (about 10 s). The substrates were cleaned by rinsing with water. The substrates were then exposed in a home-built UV-ozone chamber. This technique is well known to eliminate all organic impurities from a surface but was also used to oxidize silicon and obtain a hydrated flat silica surface free from organic pollution. The wafers were placed at a maximum distance of 5 mm from a two-wavelength low-pressure mercury lamp (λ = 185 and 254 nm) under an O2 stream. After 30 min of exposition, a hydrophilic silica surface (contact angle θ < 10°) was obtained. Its thickness, measured by ellipsometry, was about 2.5 nm and its roughness, measured by tapping mode atomic force microscopy, was about 0.15 nm.
- • Grafting of 10-isocyanatodecyltrichlorosilane on an oxidized silicon surface. The oxidized silicon wafer was placed into a Schlenk tube under a nitrogen atmosphere. The grafting solution, containing 10-isocyanatodecyltrichlorosilane (10−2 M), diisopropylethylamine (10−1 M) in trichloroethylene, was introduced into the tube. The wafer was treated with the solution for 45 min at 0 °C under nitrogen atmosphere without stirring. Then the solution was removed and the wafer was washed with trichloroethylene.
- • reaction with trianglamine 1 or 1′ or 2.
Trianglamine was dissolved in trichloroethylene (5 × 10−3 M), and the solution was reacted with SAM of isocyanates. After 30 minutes, the wafer was rinsed with trichloroethylene.
Functionalisation of the Si3N4 tip:
The tip was treated by UV-ozone in order to generate OH groups at the surface of the tip. Then the tip was immerged in a solution of isocyanatodecyltrichlorosilane 10−3 M in trichloroethylene for 45 min at 0 °C, washed with trichloroethylene, and treated by a solution of trianglamine 1′ 5 × 10−3 M in trichloroethylene for 30 min. The tip was washed with trichloroethylene.
Conflict of interest
None.
Acknowledgements
Authors thank to the BARRANDE project (reg. No. MEB 020748) for financial support.


