1 Introduction
α-olefins are important substances used extensively for the detergents, synthetic lubricants, plasticizers, alcohols and comonomers used for branched polyolefins. The linear α-olefins were first manufactured by the Ziegler (Alfen) process in the presence of triethylaluminum (TEA). The large scale process developed as the SHOP process using nickel complex catalyst in the end of 1970s [1], and was successfully industrialized in the 1980s [2–4]. From the middle of 1990s, the late-transition metal complex catalysts have attracted great attention in olefin reactivity [5]; therefore the nickel complexes in ethylene oligomerization [6,7] have been resurrected. As far as efficient catalytic systems are expected, nickel complexes bearing bidentate ligands such as N^N [8–10], N^O [11,12] and P^N [13,14] have been extensively studied. Within ferrous and cobaltous complexes acting as catalysts [15–20], various tridentate N^N^N ligands have been prepared. Interestingly, some N^N^N tridentate nickel complexes show nice activities for either ethylene oligomerization or polymerization [21–25]. Various tridentate nickel complexes have been focused in our group, and most of them showed good to high catalytic activity towards ethylene reactivity [26–30].
In order to improve catalytic activities of metal complexes and finely tune their products, the useful way is to modify ligands through different substituents on the frame of ligands. Regarding catalysts employing iminopyridines, it is common to prepare derivatives of ligands through the condensation reaction using anilines bearing different substituents. It is a challenge and more promising to have the frame of ligands substituted. Recently, we explored metal complexes ligated by 2-(benzimidazole)-6-(1-arylimino-ethyl)pyridines (Scheme 1), all of their metal (iron, cobalt or nickel) complexes showed high activities up to 107 g.mol−1 (M)·h−1 for ethylene oligomerization [20,31–36]. Considering the influence of substituents linked on to N-atom of benzimidazole, the nickel complexes containing N–H in the benzimidazole ring [34] showed better catalytic activities than their analogues containing alkyl-substituents [32,33]. Such phenomena also appeared in their analogue iron complexes [20,31,33] and chromium complexes[35,36].
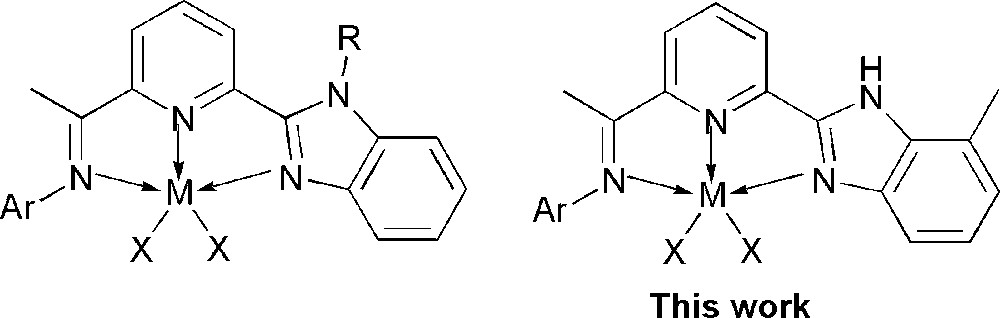
Variation of Catalyst Models.
It is very difficult to further modify the imidazole ring of benzimidazole; therefore the functionalization of benzimidazole will be fused with the benzene ring. Keeping this idea in mind, the 6-(7-methyl-1H-benzoimidazol-2-yl)-2-acetylpyridine and its isomer 6-(4-methyl-1H-benzoimidazol-2-yl)-2-acetylpyridine were newly synthesized according to our developed procedure [31]. A series of 2-(benzimidazol-2-yl)-6-(1-aryliminoethyl)pyridines was prepared and used to synthesize the nickel complexes of the title (Scheme 1). The nickel complexes showed good catalytic activities toward ethylene oligomerization using Et2AlCl as cocatalyst. The effects of substituents on the ligands, the Al/Ni molar ratio, and reaction temperature on their catalytic activities and resultant oligomers have been investigated in detail. Herein the syntheses and characterizations these title nickel complexes are reported, along with the study of their catalytic activities towards ethylene oligomerization.
2 Results and discussion
2.1 Synthesis and characterization of ligands and complexes
The starting compound 6-(methyl-substituted-1H-benzo-imidazol-2-yl)-2-acetylpyridine was synthesized according to our previous procedure (Scheme 2) [31]. There are two isomers of the product obtained, the 6-(7-methyl-1H-benzoimidazol-2-yl)-2-acetylpyridine and 6-(4-methyl-1H-benzoimidazol-2-yl)-2-acetylpyridine approximately in the ratio of 3:2 based on the 1H NMR spectrum. However, they were not separated by column chromatography. The ligands L1–L6 were prepared by treating the starting substance with corresponding aniline in toluene (Scheme 2) according to our previous procedure [37]. All of them were similarly isomerized in two configurations in the ratios about of 3:2. On the base of the 1H NMR measurement in the CDCl3 solution, ligand L5 within the range of 25 °C to 55 °C, there is no significant difference to indicate different ratios of isomes. All these compounds are stable in solid state, and fully characterized by elemental analysis, FT-IR, 1H and 13C NMR.
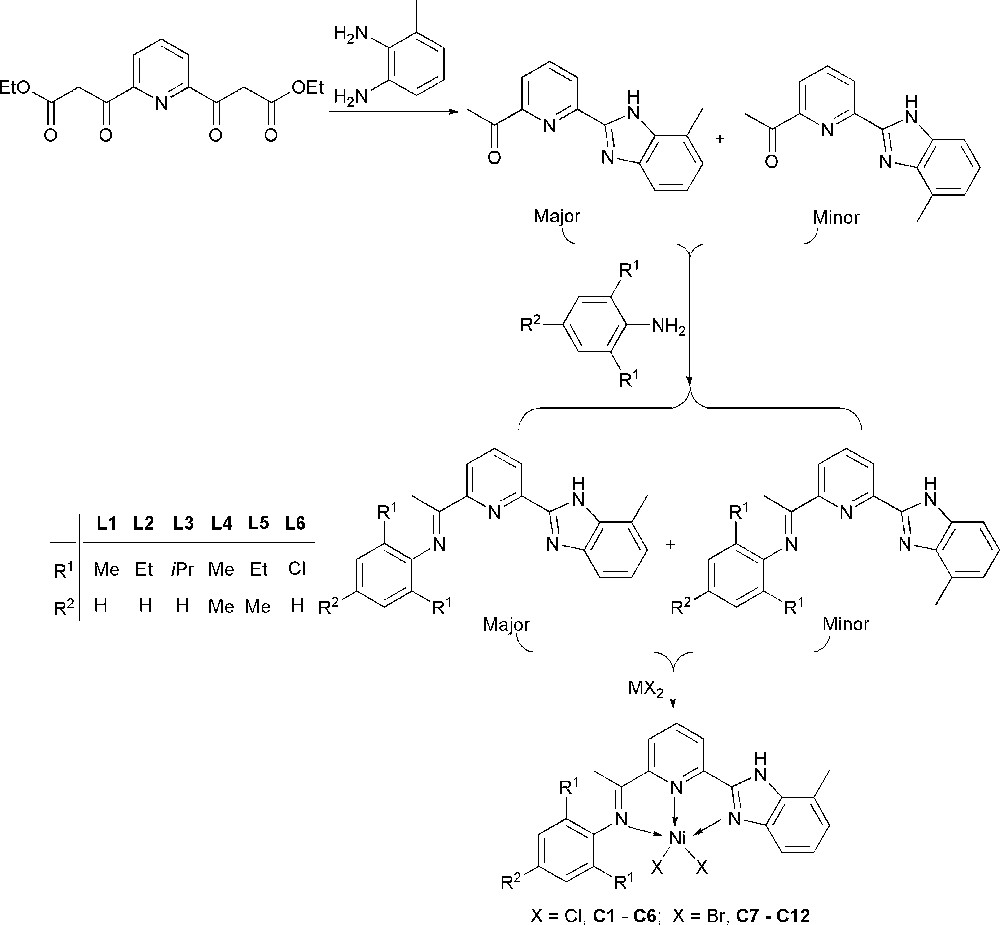
Synthesis of nickel complexes.
The dichloronickel(II) complexes (C1–C6) were prepared by the stoichiometric reactions of the appropriate ligands with NiCl2·6H2O in ethanol for 12 h. The equimolar interactions of L1–L6 with (DME)·NiBr2 in tetrahydrofuran (THF) for 12 h gave dibromonickel(II) complexes (C7–C12) (Scheme 2). All the nickel(II) complexes of the title obtained are air-stable, yellow in color and in good yields (65–95%). Their IR spectra showed absorption bands between 1590 and 1560 cm−1, which correspond to the ν (C = N) stretching frequencies about 50 cm−1 shifted to lower wavenumber in comparison with their free ligands. The single crystals of nickel complexes C1, C3 and C9 suitable for X-ray diffraction analysis were obtained by laying diethyl ether on their methanol solutions. Their complexes’ analogues reported [30–36], the dibromonickel complexes coordinate with ligands in the N^N^N tridentate manner, in which the sp2–hybridized nitrogen of benzoimidazole coordinated with nickel atom. The other isomer could not be figured out; both complexes were surprisingly observed as one isomer with methyl-substituent far away to nickel center by the X-ray diffraction analysis (Scheme 2). The favorable structures of nickel complexes were formed due to the steric hindrance of the methyl-substituent on the benzoimidazole along with its proton immigrated within the benzoimidazole.
The coordination geometry of complexes C1 and C3 could be described as distorted octahedral including one coordinated methanol, individually. In addition, there are independent methanol molecules in the crystal cell of the complex C1. The molecular structure of C1·CH3OH is shown in Fig. 1, and the selected bond lengths and angles are tabulated in Table 1. In the molecular structure of C1·CH3OH, the nickel atom deviates by 0.0182 Å from the plane formed by N(2), N(3) and N(4). The chlorine atom Cl(1) is almost in coplanar manner with deviation of 0.0947 Å, and the other chlorine atom Cl(2) deviates by 2.5367 Å from this plane in the opposite direction. The dihedral angle between the iminoaryl ring and the benzimidazole ring is 105.7°, the basal plane which formed by N(2), N(3), N(4) is nearly coplanar to the pyridine ring with the dihedral angle of 2.9°, and the dihedral angle between iminoaryl ring and the pyridine ring is 102.8°. The N(2)–C(8) (1.324(7) Å) is shorter than the N(1)–C(8) (1.356(7) Å), suggesting typical SP2–N character of the coordinated nitrogen of the benzoimidazole. The three Ni–N bond lengths are Ni(1)–N(2) 2.097(5) Å, Ni(1)–N(3) 2.013(5) Å and Ni(1)–N(4) 2.197(5) Å, respectively. Beyond the coordinated methanol with Ni(1)–O(1) (2.160(4) Å), there are slight differences of Ni(1)–Cl(1) (2.2924(17) Å) and Ni(1)–Cl(2) (2.5237(18) Å). The differences of the O–Ni–Cl angles are 92.23(13)° and 169.86(12)°.
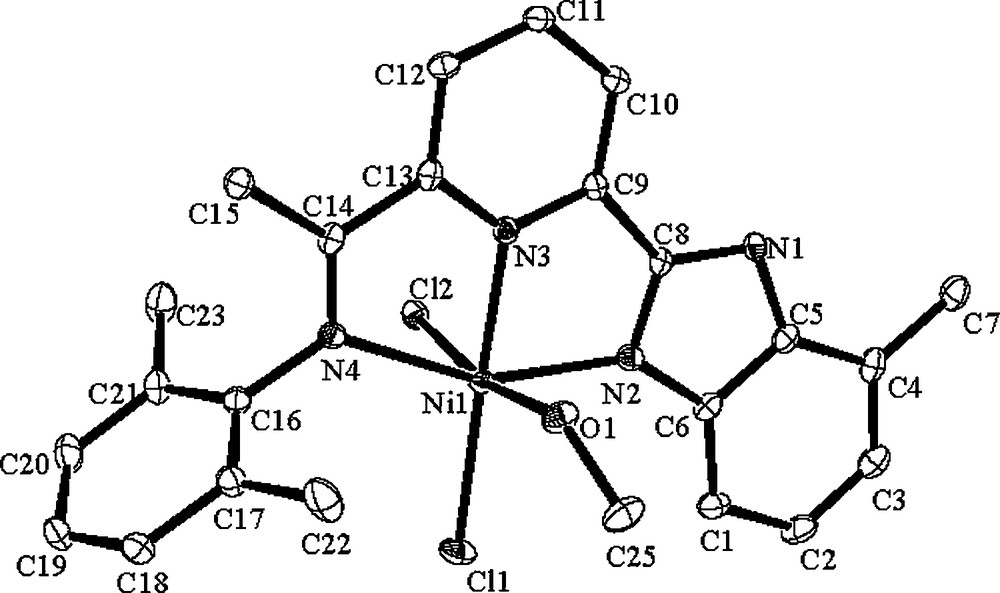
ORTEP Molecular structure of C1·CH3OH. Thermal ellipsoids are shown at 30% probability level. Hydrogen atoms have been omitted for clarity.
Selected bond lengths and Angles for C1·CH3OH and C3·CH3OH.
| C1·CH3OH | C3·CH3OH | |
| Bond lengths (Å) | ||
| Ni–N2 | 2.097(5) | 2.117(3) |
| Ni–N3 | 2.013(5) | 2.023(3) |
| Ni–N4 | 2.197(5) | 2.214(3) |
| Ni–Cl1 | 2.2924(17) | 2.3187(10) |
| Ni–Cl2 | 2.5237(18) | 2.4112(11) |
| N1–C8 | 1.356(7) | 1.357(4) |
| N2–C8 | 1.324(7) | 1.332(4) |
| N4–C14 | 1.287(8) | 1.284(4) |
| Ni–O | 2.160(4) | 2.174(3) |
| Bond angles (°) | ||
| N2–Ni–N3 | 78.74(19) | 78.30(11) |
| N2–Ni–N4 | 155.23(18) | 153.78(11) |
| N3–Ni–N4 | 76.50(18) | 75.98(11) |
| N2–Ni–Cl1 | 101.02(14) | 102.88(8) |
| N3–Ni–Cl1 | 176.65(15) | 170.83(9) |
| N4–Ni–Cl1 | 103.66(13) | 101.72(8) |
| Cl1–Ni–Cl2 | 96.45(7) | 96.51(4) |
| N2–Ni–Cl2 | 89.15(14) | 88.90(9) |
| N3–Ni–Cl2 | 86.89(14) | 92.60(9) |
| N4–Ni–Cl2 | 90.26(13) | 97.19(8) |
| O1–Ni–Cl1 | 92.23(13) | 81.82(8) |
| O1–Ni–Cl2 | 169.86(12) | 176.08(7) |
Like complex C1, the molecular structure of C3·CH3OH (Fig. 2) also shows the six-coordinated geometry with coordination of solvent methanol molecule and two Cl atoms. The selected bond lengths and angles are tabulated in Table 1. The central nickel atom deviates by 0.1235 Å from the plane containing N(2), N(3), N(4), while the Cl(1) deviates by 0.0993 Å and Cl(2) deviates by 2.5276 Å in the opposite direction. The plane composed by N(2), N(3) and N(4) is almost coplanar to the pyridyl ring with dihedral angle of 2.3°, the pyridyl ring and the benzimidazole ring are also nearly coplanar with dihedral angle of 2.8°. The dihedral angles between the phenyl plane and the benzimidazole ring, and the phenyl plane with the pyridine ring are 95.5° and 96.9°, respectively. Due to the isopropyl group in complex C3·CH3OH instead of methyl in complex C1·CH3OH (Table 1), there are slightly longer Ni–N bond lengths observed for complex C3 such as Ni(1)–N(2) 2.117(3) Å, Ni(1)–N(3) 2.023(3) Å and Ni(1)–N(4) 2.214(3) Å. The Ni–Cl bond lengths are closer: Ni(1)–Cl(1) (2.3187(10) Å) and Ni(1)–Cl(2) (2.4112(11) Å).
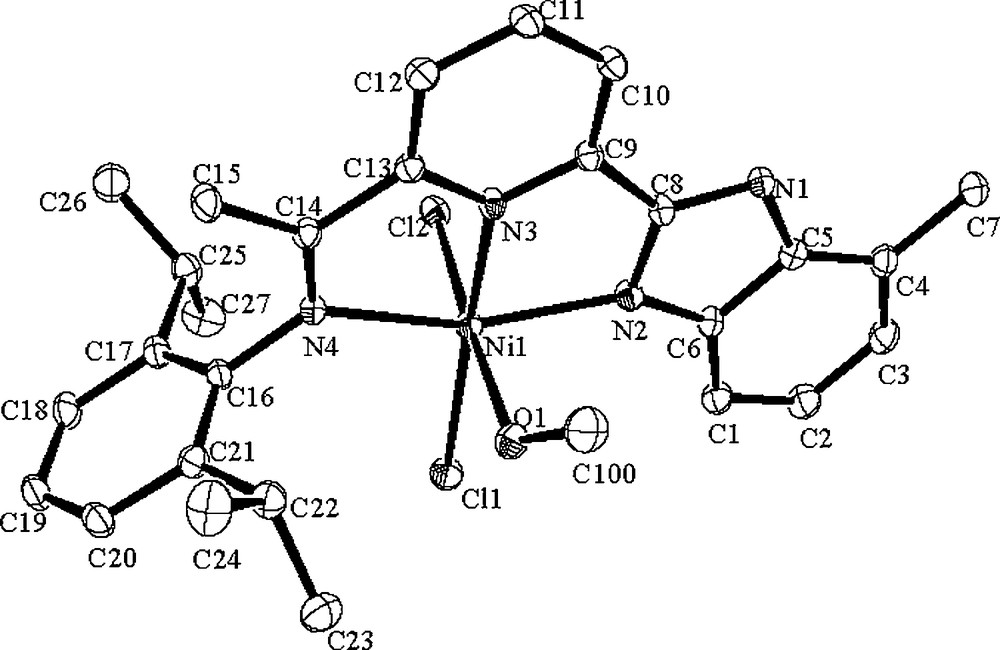
ORTEP Molecular structure of C3·CH3OH. Thermal ellipsoids are shown at 30% probability level. Hydrogen atoms have been omitted for clarity.
As shown in the Fig. 3, with the coordination of two methanol molecules, the coordination geometry of [L3NiBr2CH3OH]Br can be described as a distorted octahedron. In the single crystals of [L3NiBr2CH3OH]Br, one bromine atom of C9 was replaced by one methanol molecule and acted as a counterion which was relatively far from the nickel center. The axial plane (O(1)–Ni(1)–O(2)) is nearly perpendicular to the equatorial plane which formed by N(2), N(3) and N(4) with a dihedral angle of 91.8°. The benzimidazole ring is nearly coplanar with the pyridine ring with a dihedral angle of 2.4o. Two O-atoms (O(1) and O(2)) of coordinated methanols located in mutually trans-positions form an angle of 176.39(13)° with the nickel core and the bond lengths of the O–Ni(1) are slightly different as O(1)–Ni(1) 12.068(3) Å and O(2)–Ni(1) 2.086(3) Å. The coordination geometry was observed in its chloroanalogue ligated by 2-(1H-2-benzimidazolyl)-6-(1-(arylimino)ethyl)pyridines [34]. Compared with the chloroanalogue of [L1NiCl(CH3OH)2]Cl [34], the N–Ni bond lengths of [L3NiBr2CH3OH]Br, Ni(1)–N(2) = 2.140(3) Å, Ni(1)–N(3) = 2.016(3) Å and Ni(1)–N(4) = 2.204(3) Å, are within the typical values of coordination bonds [27,32,34].

ORTEP Molecular structure of [L3NiBr2CH3OH]Br. Thermal ellipsoids are shown at 30% probability level. Hydrogen atoms have been omitted for clarity. Selected bond lengths (Å) and angles (deg): Ni(1)–N(2) = 2.140(3), Ni(1)–N(3) = 2.016(3), Ni(1)–N(4) = 2.204(3), Ni(1)–Br(1) = 2.4742(9), Ni(1)–O(1) = 2.068(3), Ni(1)–O(2) = 2.086(3), N(2)–C(8) = 1.327(5), N(1)–C(8) = 1.348(5); N(3)–Ni(1)–N(2) = 78.46(13), N(3)–Ni(1)–N(4) = 76.14(13), N(2)–Ni(1)–N(4) = 154.57(12), O(1)–Ni(1)–O(2) = 176.39(13), N(3)–Ni(1)–O(1) = 90.82(14), N(3)–Ni(1)–O(2) = 86.99(13), O(1)–Ni(1)–N(2) = 88.73(13), O(2)–Ni(1)–N(2) = 88.03(13), O(1)–Ni(1)–N(4) = 90.38(12), O(2)–Ni(1)–N(4) = 91.87(12).
In general, all the three complexes show the six-coordinated geometry around nickel atom with coordination of additional methanol molecules. The catalytic behaviors of all complexes are investigated with the prior dried precatalysts in order to avoid any solvent incorparated. The elemental analyses of their complexes are past after being dried under vacuum more than 4 h.
2.2 Ethylene oligomerization
The nickel (II) complexes C1–C12 are systematically investigated for the oligomerization of ethylene with the Et2AlCl as a cocatalyst. Because of low catalytic activity at ambient pressure of ethylene, the screening for catalysis is carried out at 10 atm of ethylene. Complex C4 is typically investigated under a range of reaction conditions, such as molar ratios of cocatalyst to nickel and various temperature. The oligomers are only obtained and measured by GC. When the Al/Ni molar ratios of Et2AlCl to C4 are changed from 400 to 800 (Entries 1–4, Table 2), the catalytic activities first increase and then decrease. The optimum activity is observed at the Al/Ni molar ratio of 700. The influence of reaction temperatures on their catalytic activities are checked by the catalytic systems of C4 (Entries 3 and 5–7, Table 2) and C10 (Entries 21–24, Table 2). The highest activity is obtained at 30 °C (Entries 3 and 22, Table 2), and higher temperatures led to an obvious decrease of the catalytic activity. At room temperature, it is observed a little lower catalytic activity (Entries 5 and 21, Table 2) than that at 30 °C. It is assumed that the lower temperature do not activate active sites or relative lower movements of reactive substances. Meanwhile, decomposition of some active centers or unfavorable intermediates formed might happen along with a lower solubility of ethylene in toluene at higher temperature. Such phenomena have also observed in their analogue complexes [31–36]. Regarding lifetime of active species, the catalytic reactions are terminated within different reaction periods of the catalytic system of complex C4 (Entries 8–12, Table 2). The higher activities are observed in shorter reaction times; the highest in 10 mins, on the other hand, activities are decreased along with time prolongation and almost terminated after 40 mins. These results indicate that active species are immediately formed when cocatalyst is added, the catalytic activity will be slowly decreased along with prolonged reaction time and the deactivation of partial active sites due to the cumulation of impurities. Therefore, further investigations of other nickel complexes are carried out with the optimum condition at Al/Ni ratio 700 at 30 °C within 30 mins.
Ethylene catalytic activity with nickel complexes using Et2AlCla.
| Entry | Precatalyst | Al/Ni | T (°C) | t (min) | Activityc | Oligomer distributionb | ||
| C4/ΣC | C6/ΣC | α-Olefin (%) | ||||||
| 1 | C4 | 400 | 30 | 30 | 5.4 | 96.5 | 3.5 | 91.8 |
| 2 | C4 | 600 | 30 | 30 | 6.6 | 99.1 | 0.9 | 94.3 |
| 3 | Ci4 | 700 | 30 | 30 | 38 | 99.7 | 0.3 | > 99 |
| 4 | C4 | 800 | 30 | 30 | 6.6 | 96.0 | 4.0 | 96.0 |
| 5 | C4 | 700 | 20 | 30 | 26 | 90.2 | 9.8 | 92.1 |
| 6 | C4 | 700 | 40 | 30 | 23 | 91.6 | 8.4 | 50.7 |
| 7 | C4 | 700 | 50 | 30 | 11 | 95.0 | 5.0 | 83.1 |
| 8 | C4 | 700 | 30 | 10 | 52 | 99.8 | 0.2 | > 99 |
| 9 | C4 | 700 | 30 | 20 | 42 | 99.7 | 0.3 | > 99 |
| 10 | C4 | 700 | 30 | 40 | 30 | 99.7 | 0.3 | > 99 |
| 11 | C4 | 700 | 30 | 50 | 25 | 99.7 | 0.3 | > 99 |
| 12 | C4 | 700 | 30 | 60 | 21 | 99.7 | 0.3 | > 99 |
| 13 | C1 | 700 | 30 | 30 | 28 | > 99.9 | – | 91.3 |
| 14 | C2 | 700 | 30 | 30 | 25 | 99.4 | 0.6 | 98.1 |
| 15 | C3 | 700 | 30 | 30 | 17 | 94.0 | 6.0 | 77.1 |
| 16 | C5 | 700 | 30 | 30 | 35 | 94.7 | 5.3 | 21.0 |
| 17 | C6 | 700 | 30 | 30 | 8.1 | 96.3 | 3.7 | 93.3 |
| 18 | C7 | 700 | 30 | 30 | 35 | 99.3 | 0.7 | 60.1 |
| 19 | C8 | 700 | 30 | 30 | 14 | 98.9 | 1.1 | 34.6 |
| 20 | C9 | 700 | 30 | 30 | 8.3 | 97.4 | 2.6 | 12.7 |
| 21 | C10 | 700 | 20 | 30 | 31 | 97.9 | 2.1 | > 99 |
| 22 | C10 | 700 | 30 | 30 | 52 | 98.8 | 1.2 | 97.0 |
| 23 | C10 | 700 | 40 | 30 | 43 | 98.5 | 1.5 | 62.5 |
| 24 | C10 | 700 | 50 | 30 | 8.4 | 98.9 | 1.1 | 27.4 |
| 25 | C11 | 700 | 30 | 30 | 36 | 99.7 | 0.3 | > 99 |
| 26 | C12 | 700 | 30 | 30 | 5.6 | 96.6 | 3.4 | 71.0 |
a Reaction condition: 5 μmol Ni; 30 min, Et2AlCl; 10 atm ethylene; 100 mL toluene.
b Determined by GC.
c 105 g mol−1(Ni) h−1.
All nickel complexes displayed high activities and good selectivity of α-olefin with a dimer as predominated product, as summarized in Table 2. To compare the environmental influence of the ligands on their catalytic activities, the title complexes were classified into two groups based upon the anionic halides, dichlorides C1–C6 and dibromides C7–C12. The complexes C4 and C10 bearing 2,4,6-trimethyl-substituted showed the highest catalytic activity in each group (Entries 3 and 22 respectively, Table 2). Variation of the R1 groups at the ortho-positions of the imino-N aryl rings affects their catalytic performances, the increasing steric hindrance leads to decrease the catalytic activities in order of C1 > C2 > C3 (Entries 13–15, Table 2) and C7 > C8 > C9 (Entries 18–20, Table 2). With the same steric influence, the activity of complex C4 (Entry 3, Table 2) is higher than that of complex C5 (Entry 16, Table 2), and so is the activity of C10 (Entry 22, Table 2) better than that of C11 (Entry 25, Table 2). Meanwhile, complexes C6 (Entry 17, Table 2) and C12 (Entry 26, Table 2) containing ligands with chloro-substituents show lower catalytic activities. These phenomenon are caused by the solubility factors of nickel complexes, complexes with ligands containing additional methyl-substituents enhance their solubility, but complexes with ligands containing halides have lower solubility. The similar phenomena were also observed within complexes ligated by their analogues of the ligands [33,34].
Regarding the effects of anionic halides (chloride for C1–C6 and bromides for C7–C12), the synergic influences of halides and the ligands were observed for their catalytic activities. It is common for bromide precursors showing better activity than the corresponding chlorides [26,27,38], which is attributed to better solubility of the bromo complexes. In current system, ligands containing an additional methyl group generally enhance the solubilities of all complexes, therefore, the leaving groups of anionic halides synergically affect their catalytic activities with substituents of their ligands. With ortho-methyl groups of the imino-N aryl rings (Entry 13 vs. Entry 18, and Entry 3 vs. Entry 22, Table 2), the bromonickel catalysts perform better activities than these of chloronickel complexes due to the solubility of complexes. Ethyl or iso-propyl groups at ortho-positions of the imino-N aryl rings (Entry 14 vs. Entry 19, and Entry 15 vs. Entry 20, Table 2), better activities showed for chloronickel catalyst than bromonickel catalyst, which is caused by the net charge influence on catalytic acivity [39].
As previously observed [32,33], most complexes containing N–H group on the benzimidazole showed higher activities. It is imaged that some active species of anionic amides or aluminum amides formed in situ with adding organoaluminum, based on the evidences of metallocene [40,41] and complex catalysts [13]. Due to better solubility, the title complexes performed higher activities than their analogues without methyl on the phenyl ring of the benzimidazoles [34]. This is an alternative way to modify ligands with changing its solubility for improving the catalytic activities.
3 Conclusions
A series of nickel complexes was synthesized and fully characterized. The X-ray crystallographic studies on the nickel complexes C1 and C3 displayed the distorted octahedral geometry around nickel atom with incorporation of solvent molecule. All nickel complexes, activated by Et2AlCl, exhibited high activities for ethylene oligomerization. Their activities were generally higher than their analogues [34] because the title complexes, containing ligands with an additional methyl group of the benzoimidazoles, have better solubility. Within present system of ligands, the bulkier substituents decrease catalytic activities. The synergic influences of ligands and anionic halides have the considerable effects on their catalytic activities.
4 Experimental
4.1 General considerations
All manipulations of air- and moisture-sensitive compounds were carried out in nitrogen atmosphere using standard Schlenk techniques. Toluene was refluxed over sodium-benzophenone and distilled under nitrogen prior to use. Diethylaluminum chloride (Et2AlCl, 1.7 M in toluene) was purchased from Acros Chemicals. Other reagents were purchased from Aldrich or Acros Chemicals. 1H and 13C NMR spectra were recorded on a Bruker DMX 400 MHz instrument at ambient temperature using TMS as an internal standard. IR spectra were recorded on a Perkin-Elmer System 2000 FT-IR spectrometer. Elemental analysis was carried out using a Flash EA 1112 micro-analyzer. GC analysis was performed with a Varian CP-3800 gas chromatograph equipped with a flame ionization detector and a 30-m (0.2 mm i.d., 0.25 μm film thickness) CP-Sil 5 CB column. The yield of oligomers was calculated by referencing with the mass of the solvent on the basis of the prerequisite that the mass of each fraction was approximately proportional to its integrated areas in the GC trace. Selectivity for the linear α-olefin was defined as (amount of linear α-olefin of all fractions)/(total amount of oligomer products) in percent.
4.2 Preparation of the organic compounds and nickel complexes
4.2.1 Preparation of 6-(methyl-substituted-1H-benzoimidazol-2-yl)-2-acetylpyridine
According to the synthetic procedure of 6-(1H-benzoimidazol-2-yl)-2-acetylpyridine [31], a mixture of diethyl 2,6-bis(β-keto-carboxylate)pyridine [37] (9.27 g, 0.030 mol), 1.5 equivalent molar 3-methylbenzene-1,2-diamine (5.49 g, 0.045 mol), and a catalytic amount of p-toluenesulfonic acid in the 80 mL solution of toluene and isopropyl alcohol (3:1, v/v) was refluxed for 14 h. A mixture of 20 mL of acetic acid and 5 mL of concentrated HCl was added and continued to reflux for additional 6 h. The resultant solution was cooled to room temperature, a 20% KOH solution was added to neutralize the solution to the pH value between 9 and 10. The aqueous phase was extracted with ethyl acetate (20 mL × 3), all organic extracts were combined and dried over anhydrous Na2SO4. After drying and purification by column chromatography (alumina column 3/1 petroleum ether/ethyl acetate), the product obtained as white solids (3.77 g, 50.7% isolated yield) with the roughly molar ratio of 3:2 for 6-(7-methyl-1H-benzoimidazol-2-yl)-2-acetylpyridine and 6-(4-methyl-1H-benzoimidazol-2-yl)-2-acetylpyridine. For combined isomers solids: Mp: 165–166 ̊C. IR (KBr; cm−1): 3414.4 (s), 3055.7 (w), 1694.3 (s), 1591 (s), 1567 (w), 1505.6 (w), 1459.6 (m), 828.2 (s), 747.1 (s). Anal. Calcd for C15H13N3O: C, 71.70; H, 5.21; N, 16.72. Found: C, 71.42; H, 5.49; N, 16.73. 6-(7-methyl-1H-benzoimidazol-2-yl)-2- acetylpyridine: 1H NMR (400 MHz, CDCl3, TMS): δ 10.31 (s, 1H, NH), 8.65 (d, J = 3.9 Hz, 1H, Py), 8.03–8.11 (m, 1H, Py), 7.98–8.02 (m, 1H, Py), 7.40 (d, J = 8.1 Hz, 1H, Ph), 7.15–7.24 (m, 1H, Ph), 7.13 (t, J = 8.6 Hz, 1H, Ph), 2.86 (s, 3H, CH3), 2.75 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3, TMS): δ 199.3, 153.1, 149.6, 148.1, 144.1, 138.2, 133.7, 125.0, 123.3, 122.3, 117.9, 108.9, 25.9, 17.0. 6-(4-methyl-1H-benzoimidazol-2-yl)-2-acetylpyridine: 1H NMR (400 MHz, CDCl3, TMS): δ 10.19 (s, 1H, NH), 8.61 (d, J = 3.9 Hz, 1H, Py), 8.03–8.11 (m, 1H, Py), 7.98–8.02 (m, 1H, Py), 7.70 (d, J = 8.1 Hz, 1H, Ph), 7.15–7.26 (m, 1H, Ph), 7.13 (t, J = 8.6 Hz, 1H, Ph), 2.86 (s, 3H, CH3), 2.64 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3, TMS): δ 199.3, 153.1, 149.3, 148.1, 144.1, 138.2, 130.6, 125.0, 124.3, 122.5, 121.3, 108.9, 25.9, 17.2.
4.2.2 Synthesis of 2-(methyl-substituted-1H-benzoimidazol-2-yl)-6-(1-aryliminoethyl)pyridines (L1–L6)
6-[1-(2,6-dimethylphenylimio)ethyl]-2-(7-methyl-1H-benzoimidazol-2-yl)pyridine and 6-[1-(2,6-dimethylphenylimio)ethyl]-2-(4-methyl-1H-benzoimidazol-2-yl)pyridine (L1). A solution of precursor compounds (0.75 g, 3.0 mmol), 2,6-dimethylaniline (0.54 g, 4.50 mmol), and a catalytic amount of p-toluenesulfonic acid dissolved in toluene (25 mL) was refluxed for 24 h. After solvent evaporation, the crude product was purified by column chromatography on basic Al2O3 with petroleum ether/ethyl acetate (v/v, 8:1) as eluent to afford the product as a light yellow powder in 62.3% yield, with the roughly molar ratio of 3:2 for 6-[1-(2,6-dimethylphenylimio)ethyl]-2-(7-methyl-1H-benzo imidazol-2-yl)pyridine and 6-[1-(2,6-dimethylphenylimio)ethyl]-2-(4-methyl-1H-benzoimidazol-2-yl)pyridine. For combined isomers solids: Mp: 217–219 ̊C. IR (KBr; cm−1): 3641.4 (w), 3079.0 (w), 1658.3 (vs), 1595.1 (w), 1570.6 (m), 1464.1 (w), 1428.1 (m), 1313.1 (s), 822.7 (m), 755.4 (s). Anal. Calcd for C23H22N4: C, 77.94; H, 6.26; N, 15.81. Found: C, 77.58; H, 6.49; N, 15.61. 6-[1-(2,6-dimethylphenylimio)ethyl]-2-(7-methyl-1H-benzoimidazol-2-yl)pyridine: 1H NMR (400 MHz, CDCl3, TMS): δ 10.45 (s, 1H, NH), 8.57 (d, J = 5.2 Hz, 1H, Py), 8.43 (d, J = 8.0 Hz, 1H, Py), 7.97–7.99 (m, 1H, Py), 7.71 (d, J = 8.0 Hz, 1H, Py), 7.19–7.23 (m, 1H, Ph), 7.08–7.11 (m, 3H, Ph), 6.93–6.96 (m, 1H, Ph), 2.62 (s, 3H, CH3), 2.30 (s, 3H, CH3), 2.06 (s, 6H, CH3). 13C NMR (100 MHz, CDCl3, TMS): δ 166.5, 154.3, 150.7, 148.1, 138.4, 128.3, 128.2, 125.8, 123.9, 123.2, 109.0, 50.3, 18.1, 17.9, 15.2. 6-[1-(2,6-dimethylphenylimio)ethyl]-2-(4-methyl-1H-benzoimidazol-2-yl)pyridine: 1H NMR (400 MHz, CDCl3, TMS): δ 10.25 (s, 1H, NH), 8.53 (d, J = 5.5 Hz, 1H, Py), 8.43 (d, J = 8.0 Hz, 1H, Py), 7.97–7.99 (m, 1H, Py), 7.39 (d, J = 12 Hz, 1H, Ph), 7.19–7.23 (m, 1H, Ph), 7.08–7.11 (m, 3H, Ph), 6.93–6.96 (m, 1H, Ph), 2.76 (s, 3H, CH3), 2.30 (s, 3H, CH3), 2.06 (s, 6H, CH3). 13C NMR (100 MHz, CDCl3, TMS): δ 166.5, 150.7, 148.8, 148.1, 138.4, 138.1, 134.5, 128.3, 128.2, 125.8, 123.9, 123.2, 117.0, 50.1, 18.1, 17.6, 15.2.
6-[1-(2,6-diethylphenylimio)ethyl]-2-(7-methyl-1H-benzoimidazol-2-yl)pyridine and 6-[1-(2,6-diethylphenylimio)ethyl]-2-(4-methyl-1H-benzoimidazol-2-yl)pyridine (L2). Using the same procedure as for L1, L2 was obtained as a light yellow powder in 78.8% yield, with the roughly molar ratio of 3:2 for 6-[1-(2,6-diethylphenylimio)ethyl]-2-(7-methyl-1H-benzoimidazol-2-yl)pyridine and 6-[1-(2,6-diethylphenylimio)ethyl]-2-(4-methyl-1H-benzoimidazol-2-yl)pyridine. For combined isomers solids: Mp: 188–189 oC. IR (KBr; cm−1): 3641.4 (w), 3079.0 (w), 1658.3 (vs), 1595.1 (w), 1570.6 (m), 1464.1 (w), 1428.1 (m), 1313.1 (s), 822.7 (m), 755.4 (s). Anal. Calcd for C25H26N4: C, 78.50; H, 6.85; N, 14.65. Found: C, 78.23; H, 7.04; N, 14.62. 6-[1-(2,6-Diethylphenylimio)ethyl]-2-(7-methyl-1H-benzoimidazol-2-yl)pyridine: 1H NMR (400 MHz, CDCl3, TMS): δ 10. 36 (s, 1H, NH), 8.57 (d, J = 3.9 Hz, 1H, Py), 8.43 (d, J = 7.8 Hz, 1H, Py), 7.95–8.01 (m, 1H, Py), 7.71 (d, J = 8.1 Hz, 1H, Ph), 7.20–7.24 (m, 1H, Ph), 7.13–7.15 (m, 3H, Ph), 7.04–7.08 (m, 1H, Ph), 2.77 (s, 3H, CH3), 2.35–2.48 (m, 4H, CH2), 2.32 (s, 3H, CH3), 1.16 (t, J = 7.5 Hz, 6H, CH3). 13C NMR (100 MHz, CDCl3, TMS): δ 166.2, 156.0, 147.7, 144.3, 137.9, 133.6, 133.3, 130.5, 126.1, 124.0, 123.1, 122.8, 122.3, 122.1, 117.8, 108.8, 24.4, 17.1, 13.9, 13.2. 6-[1-(2,6-diethylphenylimio)ethyl]-2-(4-methyl-1H-benzoimidazol-2-yl)pyridine: 1H NMR (400 MHz, CDCl3, TMS): δ 10.27 (s, 1H, NH), 8.53 (d, J = 3.9 Hz, 1H, Py), 8.43 (d, J = 7.8 Hz, 1H, Py), 7.95–8.01 (m, 1H, Py), 7.38 (d, J = 8.1 Hz, 1H, Ph), 7.20–7.24 (m, 1H, Ph), 7.13–7.15 (m, 3H, Ph), 7.04–7.08 (m, 1H, Ph), 2.63 (s, 3H, CH3), 2.35–2.48 (m, 4H, CH2), 2.32 (s, 3H, CH3), 1.16 (t, J = 7.5 Hz, 6H, CH3). 13C NMR (100 MHz, CDCl3, TMS): δ 166.2, 156.0, 150.0, 147.6, 144.2, 137.8, 133.6, 131.3, 130.5, 126.1, 124.0, 123.6, 123.1, 122.3, 122.2, 117.8, 108.8, 24.4, 17.1, 13.9, 13.2.
6-[1-(2,6-diisopropylphenylimio)ethyl]-2-(7-methyl-1H-benzoimidazol-2-yl)pyridine and 6-[1-(2,6-diisopropylphenylimio)ethyl]-2-(4-methyl-1H-benzoimidazol-2-yl)pyridine (L3). Using the same procedureas for the L1, L3 was obtained as a light yellow powder in 81.3% yield, with the roughly molar ratio of 3:2 for 6-[1-(2,6-diisopropylphenylimio)ethyl]-2-(7-methyl-1H-benzoimidazol-2-yl)pyridine and 6-[1-(2,6-diisopropylphenylimio)ethyl]-2-(4-methyl-1H-benzoimidazol-2-yl)pyridine. For combined isomers solids: Mp: 201–202 oC. IR (KBr; cm−1): 3457.8 (m), 3058.1 (w), 2962.9 (m), 1645.0 (vs), 1591.4 (s), 1567.8 (s), 1461.3 (m), 1431.1 (m), 1316.1 (s), 905.7 (s), 755.8 (s). Anal. Calcd for C27H30N4: C, 78.99; H, 7.37; N, 13.65. Found: C, 78.61; H, 7.37; N, 13.36. 6-[1-(2,6-diisopropylphenylimio)ethyl]-2-(7-methyl-1H-benzoimidazol-2-yl)pyridine: 1H NMR (400 MHz, CDCl3, TMS): δ 10.45 (s, 1H, NH), 8.57 (d, J = 3.9 Hz, 1H, Py), 8.41–8.45 (m, 1H, Py), 7.98 (d, J = 3.7 Hz, 1H, Py), 7.25 (d, J = 4.5 Hz, 1H, Ph), 7.18–7.20 (m, 3H, Ph), 7.10–7.15 (m, 2H, Ph), 2.74–2.80 (m, 2H, CH), 2.62 (s, 3H, CH3), 2.33 (s, 3H, CH3), 1.17 (d, J = 3.9 Hz, 12H, CH3). 13C NMR (100 MHz, CDCl3, TMS): δ 166.4, 155.9, 150.3, 147.7, 144.3, 138.0, 133.6, 131.3, 126.1, 124.7, 123.6, 123.1, 122.8, 122.3, 122.2, 117.8, 108.8, 24.7, 17.1, 17.0, 13.8. 6-[1-(2,6-Diisopropylphenylimio)ethyl]-2-(4-methyl-1H-benzoimidazol-2-yl)pyridine: 1H NMR (400 MHz, CDCl3, TMS): δ 10.32 (s, 1H, NH), 8.54(d, J = 3.9 Hz, 1H, Py), 8.41–8.45 (m, 1H, Py), 7.98 (d, J = 3.7 Hz, 1H, Py), 7.38 (d, J = 4.0 Hz, 1H, Ph), 7.18–7.20 (m, 3H, Ph), 7.10–7.15 (m, 2H, Ph), 2.77 (s, 3H, CH3), 2.74–2.80 (m, 2H, CH), 2.33 (s, 3H, CH3), 1.17 (d, J = 3.9 Hz, 12H, CH3). 13C NMR (100 MHz, CDCl3, TMS): δ 166.4, 155.9, 150.1, 147.6, 144.2, 137.8, 133.6, 131.3, 126.1, 124.0, 123.6, 123.1, 122.8, 122.1, 121.3, 117.8, 108.8, 24.7, 17.2, 17.0, 13.0.
6-[1-(2,4,6-trimethylphenylimio)ethyl]-2-(7-methyl-1H-benzoimidazol-2-yl)pyridine and 6-[1-(2,4,6-trimethylphenylimio)ethyl]-2-(4-methyl-1H-benzoimidazol-2-yl)pyridine (L4). Using the same procedure as for L1, L4 was obtained as a light yellow powder in 68.7% yield, with the roughly molar ratio of 3:2 for 6-[1-(2,4,6-trimethylphenylimio)ethyl]-2-(7-methyl-1H-benzoimidazol-2-yl)pyridine and 6-[1-(2,4,6-trimethylphenylimio)ethyl]-2-(4-methyl-1H-benzoimidazol-2-yl)pyridine. For combined isomers solids: Mp: 170 oC. IR (KBr; cm−1): 3459.6 (w), 2918.5 (m), 1646.8 (vs), 1592.0 (m), 1568.5 (s), 1465.3 (m), 1428.5 (m), 1323.8 (s), 1125.0 (m), 853.6 (s), 823.5 (m), 753.2 (s). Anal. Calcd for C24H24N4: C, 78.23; H, 6.57; N, 15.21. Found: C, 78.22; H, 6.94; N, 14.95. 6-[1-(2,4,6-trimethylphenylimio)ethyl]-2-(7-methyl-1H-benzoimidazol-2-yl)pyridine: 1H NMR (400 MHz, CDCl3, TMS): δ 10.43 (s, 1H, NH), 8.56 (d, J = 3.9 Hz, 1H, Py), 8.40 (t, J = 8.1 Hz, 1H, Py), 7.96–7.98 (m, 1H, Py), 7.71 (d, J = 8.3 Hz, 1H, Ph), 7.20–7.23 (m, 1H, Ph), 7.12 (t, J = 5.9 Hz, 1H, Ph), 6.92 (s, 2H, Ph), 2.62 (s, 3H, CH3), 2.31 (s, 3H, CH3), 2.30 (s, 3H, CH3), 2.03 (s, 6H, CH3). 13C NMR (100 MHz, CDCl3, TMS): δ 166.7, 156.1, 150.5, 147.6, 146.1, 144.1, 137.8, 133.8, 132.6, 130.4, 128.8, 125.3, 124.0, 123.1, 122.8, 121.3, 117.7, 108.9, 22.8, 20.9, 18.0, 17.7, 16.8. 6-[1-(2,4,6-trimethylphenylimio)ethyl]-2-(4-methyl-1H-benzoimidazol-2-yl)pyridine: 1H NMR (400 MHz, CDCl3, TMS): δ 10.38 (s, 1H, NH), 8.52 (d, J = 4.0 Hz, 1H, Py), 8.40 (t, J = 8.1 Hz, 1H, Py), 7.96–7.98 (m, 1H, Py), 7.39 (d, J = 8.3 Hz, 1H, Ph), 7.20–7.23 (m, 1H, Ph), 7.12 (t, J = 5.9 Hz, 1H, Ph), 6.92 (s, 2H, Ph), 2.76 (s, 3H, CH3), 2.31 (s, 3H, CH3), 2.30 (s, 3H, CH3), 2.03 (s, 6H, CH3). 13C NMR (100 MHz, CDCl3, TMS): δ 166.7, 156.1, 150.2, 147.6, 146.2, 144.1, 137.7, 133.8, 132.6, 130.4, 128.8, 125.3, 124.0, 123.1, 122.8, 121.3, 117.7, 108.9, 22.8, 20.9, 18.0, 17.1, 16.7.
6-[1-(2,6-diethyl-4-methylphenylimio)ethyl]-2-(7-methyl-1H-benzoimidazol-2-yl)pyridine and 6-[1-(2,6-diethyl-4-methylphenylimio)ethyl]-2-(4-methyl-1H-benzoimidazol-2-yl)pyridine (L5). Using the same procedure as for L1, L5 was obtained as a light yellow powder in 65.3% yield, with the roughly molar ratio of 3:2 for 6-[1-(2,6-diethyl-4-methylphenylimio)ethyl]-2-(7-methyl-1H-benzo imidazol-2-yl)pyridine and 6-[1-(2,6-diethyl-4-methylphenylimio)ethyl]-2-(4-methyl-1H-benzoimidazol-2-yl)pyridine. For combined isomers solids: Mp: 200–202 oC. IR (KBr; cm−1): 3458.0 (m), 2981.4 (m), 1645.2 (vs), 1568.2 (s), 1473.2 (m), 1443.8 (m), 1316.6 (s), 1210.2 (s), 990.0 (m), 858.8 (m), 752.0 (s). Anal. Calcd for C26H28N4: C, 78.75; H, 7.12; N, 14.13. Found: C, 78.85; H, 7.18; N, 13.97. 6-[1-(2,6-diethyl-4-methylphenylimio)ethyl]-2-(7-methyl-1H-benzoimidazol-2-yl)pyridine: 1H NMR (400 MHz, CDCl3, TMS): δ 10.35 (s, 1H, NH), 8.56 (d, J = 3.9 Hz, 1H, Py), 8.41 (d, J = 7.7 Hz, 1H, Py), 7.96–7.98 (m, 1H, Py), 7.71 (d, J = 8.1 Hz, 1H, Ph), 7.20–7.24 (m, 1H, Ph), 7.12 (t, J = 6.4 Hz, 1H, Ph), 6.95 (s, 2H, Ph), 2.62 (s, 3H, CH3), 2.39–2.43 (m, 4H, CH2), 2.35 (s, 3H, CH3), 2.32 (s, 3H, CH3), 1.15 (t, J = 7.5 Hz, 6H, CH3). 13C NMR (100 MHz, CDCl3, TMS): δ 166.5, 156.1, 150.4, 147.5, 145.1, 144.1, 137.7, 133.6, 132.7, 131.0, 130.3, 126.8, 124.6, 122.9, 122.6, 122.2, 117.7, 108.8, 24.6, 21.0, 17.1, 17.0, 13.9. 6-[1-(2,6-diethyl-4-methylphenylimio)ethyl]-2-(4-methyl-1H-benzoimidazol-2-yl)pyridine: 1H NMR (400 MHz, CDCl3, TMS): δ 10.21 (s, 1H, NH), 8.52 (d, J = 3.9 Hz, 1H, Py), 8.41 (d, J = 7.7 Hz, 1H, Py), 7.96–7.98 (m, 1H, Py), 7.38 (d, J = 7.9 Hz, 1H, Ph), 7.20–7.24 (m, 1H, Ph), 7.12 (t, J = 6.4 Hz, 1H, Ph), 6.95 (s, 2H, Ph), 2.77 (s, 3H, CH3), 2.39–2.43 (m, 4H, CH2), 2.35 (s, 3H, CH3), 2.32 (s, 3H, CH3), 1.15 (t, J = 7.5 Hz, 6H, CH3). 13C NMR (100 MHz, CDCl3, TMS): δ 166.5, 156.1, 150.1, 147.5, 145.1, 144.0, 137.6, 133.6, 132.6, 131.0, 130.3, 126.7.
6-[1-(2,6-dichlorophenylimio)ethyl]-2-(7-methyl-1H-benzoimidazol-2-yl)pyridine and 6-[1-(2,6-dichlorophenylimio)ethyl]-2-(4-methyl-1H-benzoimidazol-2-yl)pyridine (L6). Using a similar procedure as for L1, but using silicic acid tetraethyl ester as a solvent instead of toluene, L6 was obtained as a white powder in 38.3% yield, with the ougly molar ratio of 3:2 for 6-[1-(2,6-dichlorophenylimio)ethyl]-2-(7-methyl-1H-benzoimidazol-2-yl)pyridine and 6-[1-(2,6-dichlorophenylimio)ethyl]-2-(4-methyl-1H-benzoimidazol-2-yl)pyridine. For combined isomers solids: Mp: 235 oC. IR (KBr; cm−1): 3464.3 (m), 3082.9 (w), 1663.0 (vs), 1593.1 (m), 1568.9 (m), 1461.8 (m), 1224.3 (m), 822.0 (m). Anal. Calcd for C21H16N4Cl2: C, 63.81; H, 4.08; N, 14.17. Found: C, 63.84; H, 4.10; N, 13.83. 6-[1-(2,6-dichlorophenylimio)ethyl]-2-(7-methyl-1H-benzo imidazol-2-yl)pyridine: 1H NMR (400 MHz, CDCl3, TMS): δ 10.36 (s, 1H, NH), 8.59 (d, 1H, J = 3.9 Hz, Py), 8.43 (d, 1H, J = 7.8 Hz, Py), 7.99–8.02 (m, 1H, Py), 7.71(d, 1H, J = 8.1 Hz, Ph), 7.39 (d, 3H, J = 8.0 Hz, Ph), 7.18–7.24 (m, 1H, Ph), 7.10–7.14 (t, 1H, J = 6.7 Hz, Ph), 7.00–7.04 (m, 1H, Ph), 2.62 (s, 3H, CH3), 2.42 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3, TMS): δ 170.8, 155.2, 150.0, 147.7, 145.5, 144.1, 137.9, 130.5, 128.3, 124.6, 124.1, 123.1, 122.7, 108.8, 17.9, 17.0. 6-[1-(2,6-dichlorophenylimio)ethyl]-2-(4-methyl-1H-benzoimidazol-2-yl)pyridine: 1H NMR (400 MHz, CDCl3, TMS): δ 10.26 (s, 1H, NH), 8.56 (d, 1H, J = 3.9 Hz, Py), 8.43(d, 1H, J = 7.8 Hz, Py), 7.99–8.02 (m, 1H, Py), 7.39 (d, 2H, J = 8.0 Hz, Ph), 7.18–7.24 (m, 1H, Ph), 7.10–7.14 (t, 1H, J = 6.7 Hz, Ph), 7.00–7.04 (m, 1H, Ph), 2.76 (s, 3H, CH3), 2.42 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3, TMS): δ 170.8, 155.2, 159.9, 147.7, 145.5, 144.1, 137.9, 133.7, 128.3, 124.6, 123.5, 122.8, 108.8, 17.9, 17.0.
4.2.3 Synthesis of tridentate nickel complexes C1–C12
Nickel complexes C1–C12 were prepared by the same synthetic procedures and obtained as a yellow powder. The synthetic procedure for C1 can be described as follows: to a mixture of ligand L1 (0.124 g, 0.35 mmol) and NiCl2. 4H2O (0.083 g, 0.35 mmol) was added freshly prepared distilled ethanol (5 mL) at room temperature. The solution turned yellow immediately. The reaction mixture was stirred for 10 h, and the precipitate was collected by filtration and washed with diethyl ether, followed by drying in vacuum. The target complex was obtained as a yellow powder in 87.6% yield. IR (KBr; cm−1): 3496.3 (w), 2975.3 (w), 1599.6 (vs), 1476.7 (m), 1424.1 (m), 1317.6 (m), 817.0 (s), 750.4 (s). Anal. Calcd for C23H22Cl2N4Ni: C, 57.07; H, 4.58; N, 11.57. Found: C, 56.77; H, 4.72; N, 11.90.
Data for C2 Yield: 84.1%. IR (KBr; cm−1): 3420.1 (w), 3046.6 (w), 2971.7 (w), 1598.8 (vs), 1449.1 (m), 1377.6 (s), 1317.7 (s), 1158.1 (m), 794.6 (s), 752.4 (s). Anal. Calcd for C25H26Cl2NiN4: C, 58.63; H, 5.12; N, 10.94. Found: C, 58.77; H, 5.17; N, 10.72.
Data for C3 Yield: 90.1%. IR (KBr; cm−1): 3390.0 (m), 3058.4 (m), 2966.0 (m), 1591.0 (vs), 1472.0 (w), 1444.9 (w), 1319.5 (s), 1202.7 (s), 816.9 (m), 766.7 (s), 746.1 (s). Anal. Calcd for C27H30Cl2NiN4: C, 60.04; H, 5.60; N, 10.37. Found: C, 60.11; H, 5.61; N, 10.22.
Data for C4 Yield: 88.7%. IR (KBr; cm−1): 3467.8 (w), 3017.4 (w), 1597.9 (vs), 1445.9 (m), 1319.1 (s), 1197.2 (s), 823.4 (m), 792.4 (s), 760.7 (m). Anal. Calcd for C24H24Cl2NiN4: C, 57.87; H, 4.86; N, 11.25. Found: C, 57.53; H, 5.10; N, 11.06.
Data for C5 Yield: 85.3%. IR (KBr; cm−1): 3282.5 (m), 3114.0 (m), 2968.5 (m), 1598.6 (vs), 1479.4 (m), 1457.0 (m), 1422.5 (m), 1316.6 (s), 1087.4 (m), 1042.4 (s), 814.6 (s), 750.5 (s). Anal. Calcd for C26H28Cl2NiN4: C, 59.35; H, 5.36; N, 10.65. Found: C, 59.00; H, 5.28; N, 10.35.
Data for C6 Yield: 67.5%. IR (KBr; cm−1): 3362.3 (m), 3084.3 (w), 1599.9 (vs), 1478.2 (w), 1409.8 (vs), 1316.6 (s), 1277.5 (s), 819.2 (m), 789.8 (s). Anal. Calcd for C21H16Cl4NiN4: C, 48.05; H, 3.07; N, 10.67. Found: C, 47.86; H, 3.43; N, 10.46.
Data for C7 Yield: 94.9%. IR (KBr; cm−1): 3268.9 (s), 1599.8 (vs), 1475.9 (m), 1420.9 (m), 1316.6 (s), 1209.6 (s), 816.4 (m), 788.5 (w). Anal. Calcd for C23H22Br2NiN4: C, 48.21; H, 3.87; N, 9.78. Found: C, 48.51; H, 3.99; N, 9.53.
Data for C8 Yield: 91.7%. IR (KBr; cm−1): 3278.9 (s), 1599.5 (vs), 1475.0 (m), 1419.2 (m), 1316.4 (s), 1271.5 (s), 868.4 (w), 793.4 (m), 747.8 (m). Anal. Calcd for C25H26Br2NiN4: C, 49.96; H, 4.36; N, 9.32. Found: C, 49.65; H, 4.17; N, 9.68.
Data for C9 Yield: 89.4%. IR (KBr; cm−1): 3381.5(m), 3054.9(w), 1597.0(vs), 1461.3(m), 1422.6(m), 1316.8(s), 1205.4(m), 816.6(m), 766.3(s). Anal. Calcd for C27H30Br2NiN4: C, 51.55; H, 4.81; N, 8.91. Found: C, 51.81; H, 5.01; N, 8.65.
Data for C10 Yield: 93.7%. IR (KBr; cm−1): 3245.7 (s), 1600.7 (vs), 1477.7 (s), 1419.8 (w), 1316.5 (s), 1238.0 (m), 1216.5 (s), 856.0 (m), 788.4 (s), 749.0 (m). Anal. Calcd for C24H24Br2NiN4: C, 49.11; H, 4.12; N, 9.54. Found: C, 49.37; H, 4.38; N, 9.25.
Data for C11 Yield: 86.8%. IR (KBr; cm−1): 3308.7 (s), 1600.4 (vs), 1476.6 (w), 1417.4 (m), 1315.5 (s), 1273.4 (m), 1212.5 (m), 814.5 (m), 788.9 (s), 749.7 (s). Anal. Calcd for C26H28Br2NiN4: C, 50.77; H, 4.59; N, 9.11. Found: C, 50.38; H, 4.63; N, 9.41.
Data for C12 Yield: 72.4%. IR (KBr; cm−1): 3278.3 (s), 3067.2 (w), 1599.8 (vs), 1466.0 (m), 1413.2 (s), 1315.1 (s), 1210.2 (m), 933.0 (s), 806.2 (s), 740.3 (m). Anal. Calcd for C21H16Br2Cl2NiN4: C, 41.09; H, 2.63; N, 9.13. Found: C, 41.28; H, 2.67; N, 8.85.
4.3 Procedure for ethylene oligomerization
Ethylene oligomerization was performed in a stainless steel autoclave (0.5 L capacity) equipped with a gas ballast through a solenoid clave for continuous feeding of ethylene at constant pressure. A 100 mL amount of toluene containing the complex and the required amount of cocatalyst was transferred into the fully dried reactor via a syringe under a nitrogen atmosphere. At the reaction temperature, the reactor was sealed and pressurized to high ethylene pressure, and the ethylene pressure was maintained during feeding of ethylene. After the reaction mixture was stirred for the desired period, the pressure was released and a small amount of the reaction solution was collected, which was then analyzed by gas chromatography (GC) to determine the composition and mass distribution of the oligomers obtained. To keep the reaction temperature constant, the autoclave is equipped with inert heat exchange tube of water.
4.4 X-ray crystallographic studies
All of the crystals of [C1·CH3OH]·CH3OH, C3·CH3OH and [L3NiBr2CH3OH]Br suitable for X-ray diffraction analysis were obtained by laying diethyl ether on a methanol solution at room temperature. With graphite-monochromated Mo Ka radiation (λ = 0.71073 Å) at 173(2) K, cell parameters were obtained by global refinement of the positions of all collected reflections. Intensities were corrected for Lorentz and polarization effects and empirical absorption. The structures were solved by direct methods and refined by full-matrix least squares on F2. All hydrogen atoms were placed in calculated positions. Structure solution and refinement were performed by using the SHELXL-97 package [42]. Details of the X-ray structure determinations and refinements are provided in Table 3. Crystallographic data have been deposited with the Cambridge Crystallographic Data Centre, CCDC 749265 ([C1CH3OH]·CH3OH), 749266 (C3CH3OH) and 755621 ([L3NiBr2CH3OH]Br), which could be obtained free of charge from The Director, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (e-mail: deposit@ccdc.cam.ac.uk; web: http://www.ccdc.cam.ac.uk).
Crystal data and structure refinement for [C1·CH3OH]·CH3OH, C3·CH3OH and [L3NiBr2CH3OH]Br.
| [C1·CH3OH]·CH3OH | C3·CH3OH | [L3NiBr2CH3OH]Br | |
| Empirical formula | C25H30Cl2N4NiO2 | C28H34Cl2N4NiO | C29H38Br2N4NiO2 |
| Formula weight | 548.14 | 572.0 | 693.16 |
| Temperature [K] | 173(2) K | 173 (2) K | 173 (2) K |
| Wavelength [Å] | 0.71073 | 0.71073 | 0.71073 |
| Crystal system | Monoclinic | Monoclinic | Triclinic |
| Space group | P2(1)/n | P2(1)/c | P-1 |
| a [Å] | 6.8170(14) | 14.330(3) | 9.3652(19) |
| b [Å] | 20.386(4) | 11.022(2) | 10.904(2) |
| c [Å] | 18.047(4) | 18.306(4) | 17.576(4) |
| α [°] | 90 | 90 | 91.64(3) |
| β [°] | 93.30 | 112.33(3) | 94.54(3) |
| γ [°] | 90 | 90 | 97.71(3) |
| V [Å3] | 2503.8(9) | 2674.5(9) | 1771.7(6) |
| Z, Dcalcd. [gcm−3] | 4, 1.454 | 4, 1.421 | 2, 1.299 |
| μ [mm−1] | 1.019 | 0.954 | 2.830 |
| F(000) | 1144 | 1200 | 708 |
| Crystal size [mm] | 0.19 × 0.07 × 0.04 | 0.20 × 0.20 × 0.20 | 0.20 × 0.20 × 0.20 |
| θ range [°] | 1.51–25.50 | 1.54–27.48 | 1.16–27.49 |
| Limiting indices | −8 ≤ h ≤ 8 | −18 ≤ h ≤ 18 | −11 ≤ h ≤ 12 |
| −24 ≤ k ≤ 24 | −14 ≤ k ≤ 14 | −14 ≤ k ≤ 14 | |
| 0 ≤ l ≤ 21 | −22 ≤ l ≤ 23 | −22 ≤ l ≤ 22 | |
| Reflections collected | 17274 | 21196 | 21779 |
| Independent reflections | 4643 [R(int) = 0.0760] | 6075 [R(int) = 0.0374] | 8084 [R(int) = 0.0368] |
| No. of parameters | 316 | 327 | 355 |
| Completeness to θ [%] | 99.8% | 99.2% | 99.4% |
| Goodness of fit on F2 | 1.258 | 1.253 | 1.113 |
| Final R indices [I > 2σ(I)] | R1 = 0.0796 | R1 = 0.0522 | R1 = 0.0562 |
| wR2 = 0.1923 | wR2 = 0.1530 | wR2 = 0.1680 | |
| R indices (all data) | R1 = 0.0905 | R1 = 0.0580 | R1 = 0.0664 |
| wR2 = 0.2005 | wR2 = 0.1660 | wR2 = 0.1763 | |
| Max./min. Δρ[a] [eÅ−3] | 0.965 and −0.557 | 0.494 and −0.611 | 1.185 and −0.863 |
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant Nos. 20942009 and 20674089) and the Foundation for Returned Overseas Chinese Scholars of Shanxi Province (2009, X. Chen).


