1 Introduction
Epoxidation of olefins is one of the fundamental oxidation reactions in industrial chemistry because epoxides serve as useful intermediates which can be used for synthesis of a wide variety of other compounds [1]. Transition metals such as rhenium [2], titanium [3], vanadium [4], manganese and molybdenum have been used for epoxidation of alkenes. Amongst them, Mo (VI) compounds are the most versatile catalysts for epoxidation of alkenes [5–10]. Molybdenum based catalysts in homogeneous phase as well as titanium catalysts in heterogeneous phase are applied for the production of propylene oxide with alkyl hydroperoxides as oxidant [11].
In recent years, the design and synthesis of catalytically active supported metal complexes have received considerable interest because these heterogeneous catalysts offer several practical advantages over their homogeneous counterparts [12–14]. In these systems, not only advantages of homogeneous catalysts such as high catalytic activity and selectivity are retained but also some properties like easier work-up, recyclability and stability of heterogeneous systems will be obtained. Different approaches have been reported for immobilization or encapsulation of molybdenum catalysts on organic polymers [15–20], silica [21–24], modified MCM-41 [25–30], and zeolites [31].
In the encapsulation, there is a direct linkage between the catalyst and the support, and it is the only method which attempts to mimic the homogeneously catalyzed reactions. Other methods, by necessity, lead to changes in the catalyst. For example, covalent tethering requires the modification of ligand (which may influence the electronic character of the ligand and/or the conformation of the ligand), and in physisorption and ion-exchange methods the catalyst should be in close proximity to the support which may also affect the electronic properties and ligand conformation.
In styrene-based copolymers, depending on the nature of the cross-linking agent, inner spaces or cavities with definite sizes are produced during the polymerization process [32,33]. For divinylbenzene (DVB) cross-linked polystyrene (PS), these cavities have a hydrophobic environment, and for hexanedioldimethacrylate (HDDMA) cross-linked PS, they have a hydrophilic environment. Molecules can be trapped in these pockets without recourse to chemical bonding. This method can be used for the encapsulation of guest molecules in a polymer if the size and geometry of the functionalized guest molecules are acceptable to the geometry of the cavities.
In this work, we report the encapsulation of molybdenum hexacarbonyl in the polystyrene cavities. This new heterogeneous catalyst, [Mo(CO)6]@PS derived catalyst, was characterized by FT-IR, UV–Vis and SEM. The activity and reusability of catalyst was investigated in the epoxidation of alkenes with tert-BuOOH (Scheme 1).
2 Experimental
All materials were commercial reagent grade and obtained from Merck and Fluka. Elemental analysis was performed on a Perkin-Elmer 2400 instrument. Atomic absorption analysis was carried out on a Shimadzu 120 spectrophotometer. FT-IR spectra were obtained as potassium bromide pellets in the range 400–4000 cm−1 with a Nicolet-Impact 400D instrument. Gas chromatography experiments (GC) were performed with a Shimadzu GC-16A instrument using a 2 m column peaked with silicon DC-200 or Carbowax 20m. In GC experiments, n-decane was used as internal standard. 1H NMR spectra were recorded on a Bruker-Avance AQS 300 MHz.
2.1 Preparation of molybdenum hexacarbonyl encapsulated in polystyrene, [Mo(CO)6]@PS derived catalyst
The monomer, i.e., styrene (S) was treated with aqueous NaOH for removing the inhibitor and stored in a refrigerator until use. Divinylbenzene (DVB) was used as the cross-linker and purified by the same method. The 2,2’-azobisisobutyronitrile (AIBN) was used as initiator. Deionized water was purged with inert gas for 1 h prior the use. In a resin kettle (250 mL) equipped with a mechanical stirrer, condenser, dropping funnel, and nitrogen inlet, poly(vinyl alcohol) (7 g, 87–89% hydrolyzed, MW = 85 000–146 000) was dissolved in water (100 mL) by vigorous stirring at 90–95 °C. The mixture was cooled to room temperature and divinylbenzene (4.8 g, 36 mmol), styrene (0.2 g, 2 mmol), and AIBN (0.1 g, 0.6 mmol) were added to the mixture. The mixture was stirred at 65 °C (650 rpm) under a gentle stream of nitrogen. A solution of Mo(CO)6 (1 g, 3.8 mmol) in 1,2-dichloroethane (5 mL, 60 °C) was added to the reaction mixture during polymerization. The polymerization was continued with vigorous stirring under a gentle stream of nitrogen for 24 h. At the end of polymerization reaction, the mixture was cooled to room temperature and [Mo(CO)6]@PS was washed by repeated sedimentation from water at 10 °C in a centrifuge (13 000 rpm) for 30 min. Finally, the beads were dried in a vacuum oven at 80 °C overnight [34].
2.2 General procedure for epoxidation of alkenes
To a 25 mL round bottom flask equipped with a magnetic stirring bar, alkene (0.25 mmol), TBHP (0.5 mmol), [Mo(CO)6]@PS derived catalyst (0.02 mmol) and 1,2-dichloroethane (4 mL) were mixed and refluxed. The reaction progress was monitored by GC. At the end of the reaction, Et2O (20 mL) was added and filtered. The resin was thoroughly washed with Et2O and combined washings and filtrates were purified on a silica-gel plate or a silica-gel column. IR and 1HNMR spectral data confirmed the identities of the products. Blank experiment in the presence of an oxidant and using the same experimental conditions in the absence of a catalyst was also performed.
2.3 Reusability of the catalyst
The reusability of [Mo(CO)6]@PS derived catalyst was studied in the repeated epoxidation reaction of cis-cyclooctene. The reactions were carried out as described above. At the end of each reaction, the mixture was filtered, washed with Et2O, CHCl3, THF and (CH3)2CO, dried in an oven at 80 °C and reused.
3 Results and discussion
3.1 Preparation and characterization of the catalyst
The [Mo(CO)6]@PS derived catalyst was prepared via the known procedure for the suspension polymerization (Scheme 2). This may be the most well-known method of polymerization, and involves the continuous addition of monomer units to a growing free-radical chain. Initiation is a two-stage process in which, first a free radical is formed, and then this radical is added to a monomer unit. The second stage is essentially similar for all related processes; however, the first step can be achieved in a variety of ways; and the type of initiator depends on the nature of the polymerization experiment. In this method, 2,2’-azo-bisisobutyronitrile (AIBN) is the initiator of this free-radical process presumably because of the convenient timescale of its decomposition at 60 °C. A solid framework is formed during the polymerization using a mixture of monomers in the presence of a molecular template and a small volume of a porogenic solvent. A large excess of cross-linker ensures that the solid framework that is formed around each molecule of template is relatively rigid, such that the presence of the template creates a “cavity” in the polymer structure.

Preparation of [Mo(CO)6@PS] derived catalyst.
The prepared catalyst was characterized by FT-IR, SEM, diffuse reflectance UV-vis and elemental analysis.
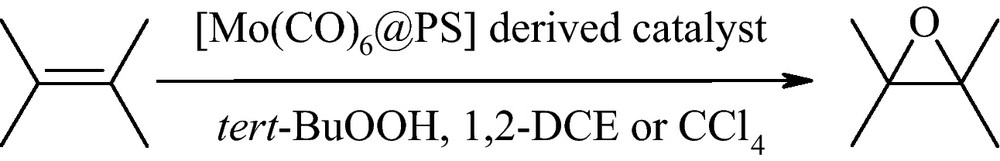
The FT-IR spectra of cross-linked PS and [Mo(CO)6]@PS derived catalyst are shown in Fig. 1A and1B, respectively. The peaks at 1981 and 502 cm−1 in the FT-IR spectrum of [Mo(CO)6]@PS derived catalyst (Fig. 1B) are attributed to C = O and Mo − CO stretching bands, respectively. These bands do not observe in the FT-IR spectrum of cross-linked PS (Fig. 1A).
The FT-IR spectra of: (A) Crosslinked PS; and (B) [Mo(CO)6]@PS derived catalyst.
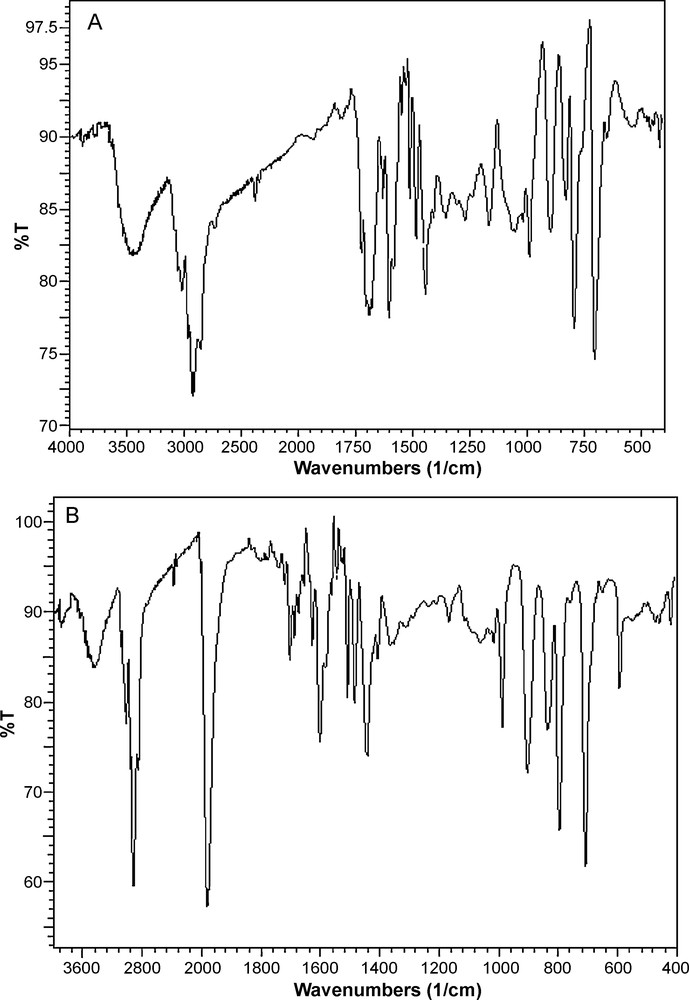
Fig. 2 shows the SEM images of cross-linked polystyrene and [Mo(CO)6]@PS derived catalyst. The images clearly indicate that the prepared resins have different morphologies.
The SEM images of: (A) Crosslinked PS and (B) [Mo(CO)6]@PS derived catalyst.
UV-Vis spectroscopy was employed in the diffuse reflectance mode for the encapsulated catalyst and cross-linked polystyrene. The latter exhibits a broad band between 200–400 nm, whereas the former shows bands at 230 and 286 nm regions which can be attributed to MLCT bands (Fig. 3). These observations clearly indicates that the Mo(CO)6 moiety has been encapsulated in the polymer network.

The diffuse reflectance UV-Vis spectra of: (A) Crosslinked PS and (B) [Mo(CO)6]@PS derived catalyst.
The metal loading of [Mo(CO)6]@PS derived catalyst, determined by atomic absorption spectroscopy (AAS), was obtained 0.52 mmol/g.
3.2 The effect of solvent on the epoxidation of cyclooctene with tert-BuOOH catalyzed by [Mo(CO)6]@PS derived catalyst
First, the effect of different solvents on the catalytic activity of the resulting catalyst was investigated in the epoxidation of cis-cyclooctene with tert-butylhydroperoxide. Amongst 1,2-dichloroethane, chloroform, carbon tetrachloride, acetonitrile, acetone and tetrahydrofurane, 1,2-dichloroethane and carbon tetrachloride were the suitable solvents because the highest epoxide yield was observed (Table 1). It seems that non-coordinate solvents such as chlorinated ones are the best solvents for oxidation reactions by molybdenum based catalysts.
The effect of different solvents on the epoxidation of cis-cyclooctene with tert-BuOOH catalyzed by [Mo(CO)6]@PS derived catalyst under reflux conditionsa.
| Row | Solvent | Epoxide (%)b | Time (h) |
| 1 | CH3CN | 30 | 2.5 |
| 2 | (CH3)2CO | 5 | 2.5 |
| 3 | THF | 6 | 2.5 |
| 4 | CHCl3 | 27 | 2.5 |
| 5 | CCl4 | 96 | 2.5 |
| 6 | 1,2-Dichloroethane | 96 | 1.5 |
a Reaction conditions: cis-cyclooctene (0.25 mmol), tert-BuOOH (0.5 mmol), catalyst (0.020 g, 0.01 mmol Mo), solvent (4 mL).
b GC yield based on the starting cyclooctene.
3.3 The effect of different oxidants on the epoxidation of cyclooctene catalyzed by [Mo(CO)6]@PS derived catalyst
We also investigated the ability of different oxygen donors such as tert-BuOOH, NaIO4 and H2O2 in the oxidation of cis-cyclooctene, and TBHP was chosen as oxygen donor (Table 2).
The effect of different oxidants on the epoxidation of cis-cyclooctene by [Mo(CO)6@PS] derived catalyst under reflux conditionsa.
| Row | Oxidant | Solventb | Epoxide(%)c | Time(h) |
| 1 | TBHP | CCl4 | 96 | 2.5 |
| 2 | 1,2-DCE | 96 | 1.5 | |
| 3 | NaIO4 | CCl4/H2Od | No Reaction | 2.5 |
| 4 | CH3CN/H2O | No Reaction | 2.5 | |
| 5 | H2O2 | CCl4 | 4 | 2.5 |
| 6 | CH3CN | 5 | 2.5 |
a Reaction conditions: cis-cyclooctene (0.25 mmol), tert-BuOOH (0.5 mmol), catalyst (0.020 g, 0.01 mmol Mo).
b A 3:1 mixture of organic solvent: water was used.
c GC yield based on the starting cyclooctene.
d Tetrabutylammonium bromide (0.01 g) was used.
3.4 Catalytic alkene epoxidation with tert-BuOOH in the presence of [Mo(CO)6]@PS derived catalyst
Since homogeneous molybdenum complexes are not recoverable and reusable, therefore, we decided to design a new catalytic system based on the heterogenization of Mo(CO)6 by its encapsulation in the polystyrene network, [Mo(CO)6]@PS derived catalyst, which gives a heterogeneous catalyst with high Mo loading.
Under the optimized conditions, the epoxidation of different alkenes with TBHP was carried out in the presence of [Mo(CO)6]@PS derived catalyst in 1,2-dichloroethane (Table 3) and carbon tetrachloride (Table 4). This catalyst efficiently catalyzed the epoxidation of both cyclic and linear alkenes with TBHP. 1-Octene and 1- dodecene as linear alkenes were efficiently converted to their corresponding epoxides by [Mo(CO)6]@PS derived catalyst in both solvents. Cyclooctene was also epoxidized in high yield with 100% selectivity both in CCl4 and 1,2-DCE. But very different results were obtained for oxidation of cyclohexene, styrene and α-methylstyrene. The epoxide selectivities for cyclohexene were 90 and 69% in CCl4 and 1,2-DCE, respectively. In the case of styrene and α-methylstyrene, the conversions in CCl4 were 95 and 80% with 81 and 69% selectivity, respectively, while in 1,2-DCE, both conversions and selectivities were lower.
Epoxidation of alkenes with tert-BuOOH catalyzed by [Mo(CO)6@PS] derived catalyst in 1,2-DCE under reflux conditionsa.
| Entry | Alkene | Conversion (%)b | Epoxide selectivity (%) | Time (h) |
| 1 | 96 | 100 | 1.5 | |
| 2 | 42 | 69 | 3 | |
| 3 | 28 | 43 | 3.5 | |
| 4 | 58 | 26 | 5.5 | |
| 5 | 84 | 100 | 7 | |
| 6 | 78 | 100 | 10 |
a Reaction conditions: alkene (0.25 mmol), tert-BuOOH (0.5 mmol), catalyst (0.020 g, 0.01 mmol Mo) and 1,2-dichloroethane (4 ml).
b GLC yield based on starting alkene.
Epoxidation of alkenes with tert-BuOOH catalyzed by [Mo(CO)6@PS] derived catalyst in CCl4 under reflux conditionsa.
| Entry | Alkene | Conversion (%)b | Epoxide Selectivity (%) | Time (h) |
| 1 | 96 | 100 | 2.5 | |
| 2 | 49 | 90 | 7 | |
| 3 | 95 | 81 | 3 | |
| 4 | 80 | 69 | 5 | |
| 5 | 93 | 100 | 3.5 | |
| 6 | 87 | 100 | 10 |
a Reaction conditions: alkene (0.25 mmol), tert-BuOOH (0.5 mmol), catalyst (0.020 g, 0.01 mmol Mo) and CCl4 (4 ml).
b GLC yield based on starting alkene.
3.5 Catalyst reusability
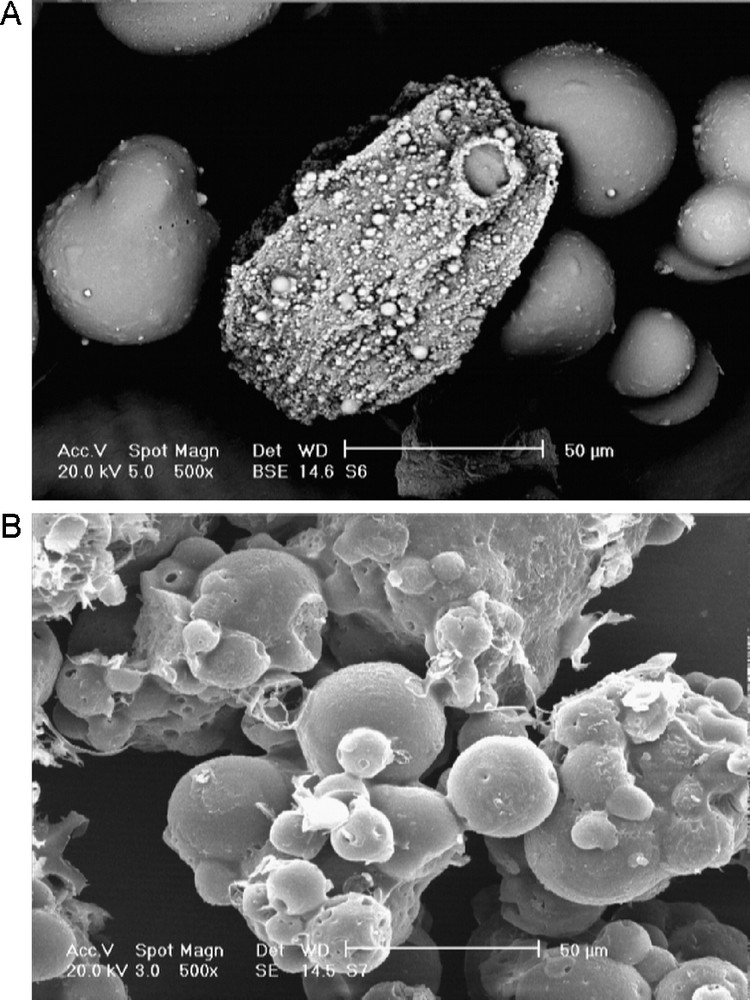
The reusability of the catalysts was monitored using multiple sequential epoxidation of cis-cyclooctene with TBHP (Table 5). To assess long-term stability and reusability of [Mo(CO)6]@PS derived catalyst, epoxidation of cis-cyclooctene was chosen as model reaction, and recycling experiments were carried out with a single sample of the catalyst. After each experiment, the catalyst was removed by simple filtration, washed with Et2O and dried in an oven at 50 °C and reused. After reusing the catalyst for ten consecutive times, the conversion was 90% (Table 5). No molybdenum was detected in the filtrates by ICP measurement after first run. The nature of the recovered catalyst was studied by FT-IR spectra, in which the CO bands have disappeared in its FT-IR spectra (Fig. 4). On the other hand, the DR UV-vis of the recovered catalyst (Fig. 5) is different from initial catalyst which is in accordance with FT-IR spectrum. The mechanism of these reactions has been previously reported [35,36]. Upon reaction of [Mo(CO)6] with TBHP, the Mo = O species are produced and act as catalytic active species. The appearance of a band at 1071 cm−1 in the FT-IR of recovered catalyst can be attributed to the presence of Mo = O species. Therefore, [Mo(CO)6] may be considred as a catalyst precursor.
Reusability of [Mo(CO)6]@PS derived catalyst in the epoxidation of cyclooctene in the presence of TBHP under reflux conditionsa.
| Run | Epoxide (%)b | Time (h) | Mo leached (%)c |
| 1 | 96 | 1.5 | 2 |
| 2 | 92 | 1.5 | 0 |
| 3 | 91 | 1.5 | 0 |
| 4 | 91 | 1.5 | 0 |
| 5 | 91 | 1.5 | 0 |
| 6 | 91 | 1.5 | 0 |
| 7 | 91 | 1.5 | 0 |
| 8 | 91 | 1.5 | 0 |
| 9 | 91 | 1.5 | 0 |
| 10 | 91 | 1.5 | 0 |
a Reaction conditions: alkene (0.5 mmol), tert-BuOOH (1 mmol), catalyst (0.010 g of supported catalyst, 0.016 mmol Mo) and 1,2-dichloroethane (3 ml).
b GLC yield based on the starting alkene.
c Determined by ICP.
The FT-IR spectrum of recovered catalyst.
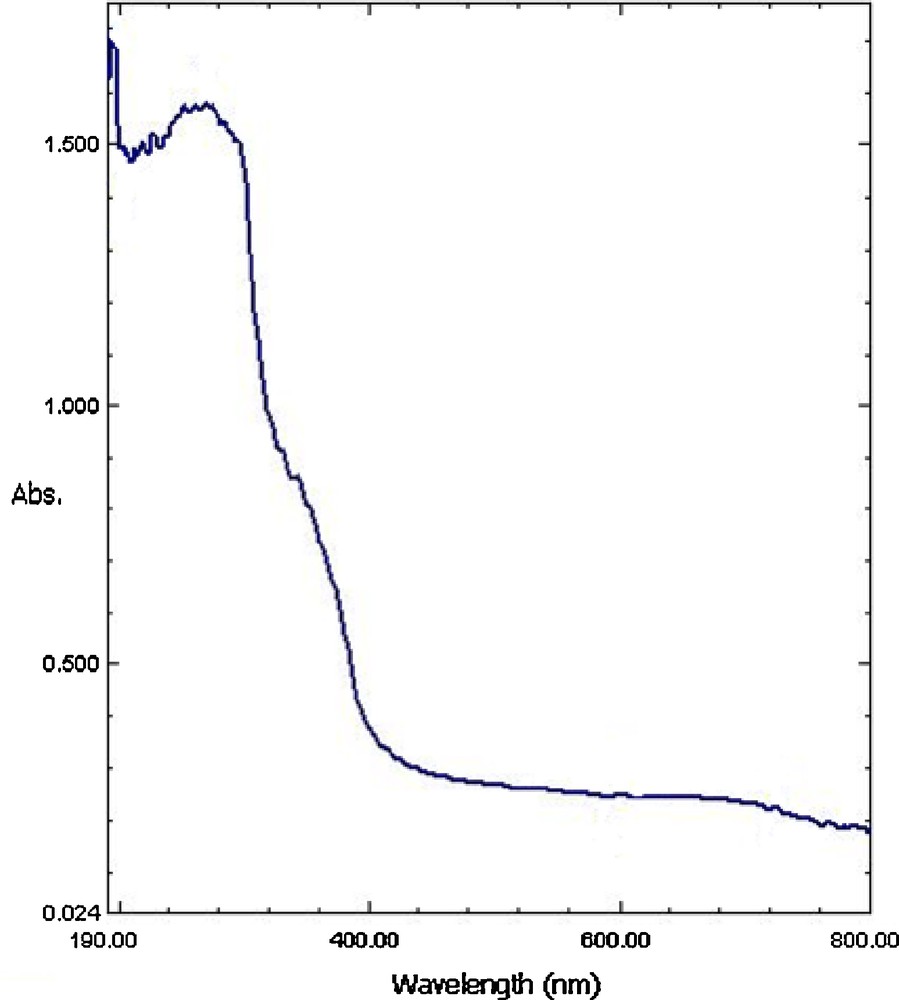
The diffuse reflectance UV-Vis spectrum of recovered catalyst.
4 Conclusion
In conclusion, molybdenum hexacarbonyl encapsulated into polystyrene, Mo(CO)6@PS derived catalyst, which was prepared by suspension polymerization of styrene and divinylbenzene in the presence of Mo(CO)6 derived catalyst, was used as an efficient catalyst for epoxidation of a wide variety of alkenes, including aromatic and aliphatic terminal alkenes using tert-butylhydroperoxide. The new heterogenized molybdenum carbonyl epoxidation catalyst was recovered by simple filtration and showed no appreciable loss of activity even after ten times of recycling.


