1 Introduction
Recently, synthetic chemists have focused their attention towards the transformations involving C-P bond formation [1]. In this endeavor Michaelis-Arbuzov [2], Michaelis-Becker [3] and phospha-Michael addition [1] are most prominent because of the versatile chemistry of their products. Amongst these, the phospha-Michael addition, i.e. the addition of a phosphorus nucleophile to an acceptor-substituted alkene or alkyne represents one of the most versatile and powerful tools for the formation of a C-P bond since many different electrophiles and phosphorus nucleophiles can be combined with each other. This offers the possibility to access many diversely functionalized products.
One of the important application of the phospha-Michael addition is the synthesis of (2-amino-3-cyano-4H-chromen-4-yl) phosphonic acid diethyl ester, which retains the basic skeleton of 2-amino chromene. This skeleton is an important structural motif in a series of natural products [4] and can be converted into pyridine systems which relate to pharmacologically important calcium antagonists of the dihydropyridine[DHP] [5] type. Along with this, it accounts an important application in the field of drugs and pharmaceuticals as anti-coagulant, diuretic, spasmolytic, anticancer and anti-anaphylactin agent [6]. Also, it can be used as cognitive enhancer for the treatment of neurodegenerative disease including Alzheimer's disease, amyoprophic lateral sclerosis, Huntington's disease, Parkinson's disease, AIDS associated dementia, and Down's syndrome as well as for the treatment of schizophrenia and myoclonus [7]. Besides biological significance, some 2-aminochromenes have been widely used as photoactive materials [8] which led the scientific community to synthesize derivatives of 2-aminochromens.
Recently, the synthesis of novel derivatives of 2-aminochromens, viz (2-amino-3-cyano-4H-chromen-4-yl) phosphonic acid diethyl esters, was carried out by Perumal et al. using InCl3 [9] as Lewis acid catalyst at ambient temperature as well as by Nageswar et al. using β-cyclodextrin [10] at 60–70 °C. The thorough scrutiny of literature revealed that these are the only reports on synthesis of (2-amino-3-cyano-4H-chromen-4-yl) phosphonic acid diethyl ester. Hence, an economical protocol with an easily available basic catalyst operable at ambient temperature for synthesis of (2-amino-3-cyano-4H-chromen-4-yl) phosphonic acid diethyl ester is highly desired.
2 Result and discussion
In our continuous interest in potassium phosphate as well as synthesis of pyrans [11,12]. We would like to report a mild, cost-effective procedure for the one-pot multi-component synthesis of (2-amino-3-cyano-4H-chromen-4-yl) phosphonic acid diethyl esters (4) by the condensation of salicylaldehydes (1), malononitrile (2) and triethyl phosphite (3) in the presence of a catalytic amount of potassium phosphate at ambient temperature in ethanol medium (Scheme 1).
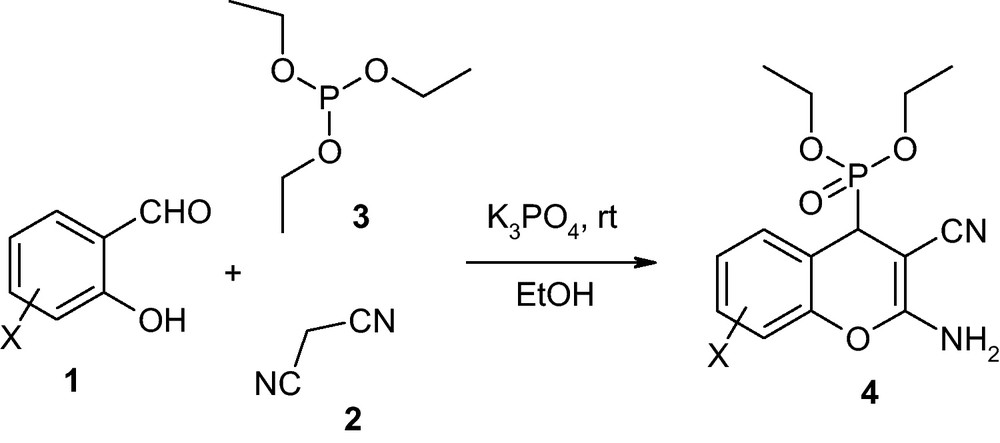
Multi-component synthesis of 2-amino-3-cyano-4H-chromen-4-yl) phosphonic acid diethyl ester.
We envisioned that K3PO4 could be a suitable catalyst for the present transformation, since the anion PO43− is sufficiently basic for the formation of cyanoolefin (5) followed by ring closure via nucleophilic attack of the OH group on the cyano group to form imino coumarin (6) and subsequent phospha-Michael addition of triethyl phosphite (3) affording the target compound (4). A plausible mechanism for the multi-component synthesis of (2-amino-3-cyano-4H-chromen-4-yl) phosphonic acid diethyl ester using K3PO4 in EtOH is depicted in Scheme 2.

A plausible mechanism for formation of 2-amino-3-cyano-4H-chromen-4-yl) phosphonic acid diethyl ester.
In an initial study, optimization of the amount of K3PO4 as well as the solvent effect were examined by the reaction of 1 (1 mmol), 2 (1 mmol) with 3 (1 mmol) in different solvents (Table 1). The best conditions employ K3PO4 as a catalyst, 1, 2, 3 in a 0.20:1:1:1 mole ratio at room temperature using EtOH as a solvent. It is worthy of note that in solvents like H2O, MeCN and MeOH (Table 1, entries 1,3,4) a mixture of products 4 and 6 (Scheme 3) was obtained while in CHCl3 there is no progress in reaction after 3 h (Table 1, entry 2) under the same reaction conditions. The catalytic activity of other catalysts generating K+ and PO43− ions such as K2HPO4, and KH2PO4 is also tested and we found that they resulted in inferior results, 74 and 66% yield of product respectively under the same conditions. The experimental procedure for this reaction is remarkably simple and synthesis could not be achieved in absence of catalyst even though reflux condition was applied for 5 h (entry 8, Table 1).
Effect of solvent and optimization of amount of K3PO4 on the yield of product 4.
| Entry | Solventa | Product | Catalyst (Mol %) | Time (min) | Yield (%) |
| 1 | H2O | 4 + 6 | 20 | 120 | 28b + 56c |
| 2 | CHCl3 | ----- | 20 | 180 | ---- |
| 3 | MeCN | 4 + 6 | 20 | 120 | 72b + 12.8c |
| 4 | MeOH | 4 + 6 | 20 | 65 | 47b + 41c |
| 5 | EtOH | 4 | 10 | 70 | 81 |
| 6 | EtOH | 4 | 15 | 60 | 86 |
| 7 | EtOH | 4 | 20 | 40 | 90 |
| 8 | EtOH | ----- | --- | 300 | --- |
a Reaction conditions: Salicylaldehyde (1 mmol), malononitrile (1 mmol), triethylphosphite (1 mmol), solvent (5 mL), temp.: r.t.
b Corresponds to yields of product 4 by LCMS.
c Corresponds to yields of product 6 by LCMS.

Mixture of products obtained in H2O, MeCN and MeOH.
Under these optimized conditions, the reaction between various substituted salicylaldehydes (1), 2 and 3 in the presence of potassium phosphate were investigated (Scheme 1, Table 2). It was found that all the reactions proceed smoothly to give the corresponding phosphonic acid diethyl esters in high yields (Table 2). Both salicylaldehyde bearing electron-donating substituents (entries 4c–4e) and electron-withdrawing groups (entries 4b, 4f–4i) gave excellent yields. The mildness of the procedure makes it very selective, as it tolerates a variety of functionalities, including chloro, bromo, methyl, methoxy, nitro, and naphthyl groups. The results are summarized in Table 2, which clearly demonstrates the generality and scope of the protocol.
Potassium phosphate catalyzed multicomponent synthesis of (2-amino-3-cyano-4H-chromen-4-yl) phosponic acid diethyl ester at room temperature.
| Entry | Product (4) | Time (min) | Yield (%)a,b |
| a | 40 | 90 | |
| b | 30 | 93 | |
| c | 35 | 88 | |
| d | 45 | 81 | |
| e | 45 | 88 | |
| f | 35 | 94 | |
| g | 20 | 89 | |
| h | 55 | 79 | |
| i | 30 | 95 | |
| j | 55 | 74 |
a All products showed satisfactory spectroscopic data (IR, 1H, 13C and 31P NMR).
b Yields refer to pure, isolated products.
3 Conclusion
In summary, we have described a practical method for the rapid synthesis of (2-amino-3-cyano-4H-chromen-4-yl) phosphonic acid diethyl ester using potassium phosphate as an inexpensive catalyst at ambient temperature. High yields along with simple reaction conditions as well as easy work-up procedure auger well for the application of this strategy for the synthesis of (2-amino-3-cyano-4H-chromen-4-yl) phosphonic acid diethylester.
4 Experimental
4.1 General
IR spectra were recorded on a Perkin–Elmer FT-IR 783 spectrophotometer. NMR spectra were recorded on a BrukerAC-400 spectrometer in DMSO-d6 using tetramethylsilane as internal standard. Mass spectra (LCMS) were recorded on Shimadzu LCMS 2010, mass spectrometer. Melting points are uncorrected.
4.2 Typical procedure
A mixture of salicylaldehyde (1 mmol), malononitrile (1 mmol), triethylphosphite (1 mmol) and K3PO4 (20 mol %) in ethanol (5 mL) was stirred at r.t. till the completion of reaction as monitored by TLC. (Table 2). Then the reaction mixture was poured into ice water and just filtered to yield corresponding (2-amino-3-cyano-4H-chromen-4-yl) phosphonic acid diethyl ester.
4.3 Spectral data of representative compounds
Entry 4e: mp 172–174 ̊C; IR (KBr): 3342, 3160, 2979, 2191, 1658, 1499, 1232, 1040, 971 cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 1.16 (t, 3H, CH3, J = 7.2 Hz), 1.21 (t, 3H, CH3, J = 7.2 Hz), 3.73 (s, OCH3), 3.95 (m, 4H, -CH2), 4.05 (d, 1H, 2JPH = 18 Hz), 6.82 (t, 1H, J = 2.4 Hz), 6.86 (dt, 1H, J = 2.4, J = 9.2 Hz), 6.96 (d, 1H, J = 8.8 Hz), 7.07 (bs, 2H, -NH2, D2O exchangeable); 13C NMR (100 MHz, DMSO-d6): δ 16.1, 16.2, 34.1, 35.5, 46.9, 55.4, 62.1, 62.2, 114.0, 116.6, 118.6, 120.1, 143.8, 155.3, 162.8; 31P-NMR (162 MHz, DMSO-d6): δ 23.39.
Entry 4g: mp 169–170 ̊C; IR (KBr): 3444, 3343, 2891, 2194, 1646, 1426, 1248, 1034, 864 cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 1.14 (t, 3H, CH3, J = 7.2 Hz), 1.18 (t, 3H, CH3, J = 7.2 Hz), 3.98 (m, 4H, -CH2), 4.25 (d, 1H, 2JPH = 18.8 Hz), 7.27 (t, 1H, J = 2.4 Hz), 7.38 (bs, 2H, -NH2, D2O exchangeable), 7.64 (t, 1H, J = 2.4 Hz); 13C NMR (100 MHz, DMSO-d6): δ 16.1, 33.9, 35.3, 47.3, 62.3, 62.5, 119.2, 121.2, 121.7, 127.7, 127.9, 128.5, 144.8, 161.8; 31P-NMR (162 MHz, DMSO-d6): δ 22.47.
Acknowledgement
Authors DMP and KAU thank UGC, New Delhi for financial assistance [F.2-3/2007(Policy/SR)] and for a Research Fellowship, respectively.


